
Chemists have long explored hydrazide compounds searching for building blocks in pharmaceuticals and material science. Succinic dihydrazide surfaced from these efforts, drawing attention after the emergence of hydrazine-based chemistry in the early 20th century. Folks in research labs during this era valued new nitrogen-rich molecules for both their reactivity and potential uses. Succinic dihydrazide didn’t take the spotlight overnight. Texts from the mid-1900s referenced it as a promising candidate for modern synthesis. By the 1970s, manufacturers started scaling up its production, activating interest from polymer science to agrochemical companies, and it eventually became a staple in certain specialty industries.
Succinic dihydrazide has worked its way from specialty chemical to regular staple in labs addressing everything from crosslinking to novel drug intermediates. Recognized by the chemical formula C4H10N4O2, its two hydrazide groups help push its reactivity beyond simple diamides. The white crystalline powder might seem unassuming, but those who have used it in condensation reactions or polymer modifications know just how much leverage those groups give. For people seeking a reagent that bridges organic synthesis and fine chemicals, few chemicals offer quite the same blend of stability and utility.
Succinic dihydrazide stands out for its solid, white, crystalline appearance. Individuals gripping a flask of this compound notice it doesn’t have much of an odor, which helps with benchwork. Its melting point lands just above 180°C, so it doesn’t decompose easily with routine heating, another reason why researchers don't need to worry much about heat during standard reactions. On the solubility front, it dissolves in water and many alcohol solvents. Chemically, it holds a moderate molecular weight of about 146.15 g/mol, and those dual hydrazide groups offer ample opportunity for condensation and coupling reactions. The compound resists oxidation under normal lab conditions, which lets chemists store it for months without degradation. Its structure brings not only predictable basicity but also a certain toughness, holding up against many organic solvents and acidic environments alike.
Quality-conscious manufacturers don’t take shortcuts on specifications. Succinic dihydrazide should reach purity of at least 98% for most research and industrial applications. Analysts in quality control routinely run FTIR and NMR spectroscopies to confirm identity and absence of major impurities. Shelf-stable packaging, usually high-density polyethylene bottles, keeps moisture out and prevents caking. Labels list batch number, production date, and technical data such as melting point and molecular weight. The safety section gets special attention, given the nitrogen content, which means suppliers must include hazard codes and advice on inhalation risk. On the bulk scale, shipment documents track origins, lot consistency, and storage histories, giving downstream users confidence in reproducibility.
Industrial-scale synthesis generally relies on the reaction between succinic acid diethyl ester and hydrazine hydrate. Starting with the diester allows for better control over reaction exotherms. Chemists charge the reaction flask with the diester and add hydrazine hydrate under cooled conditions to manage outgassing. After introducing the hydrazine, the mixture stirs under reflux for several hours, at which point the dihydrazide separates as a crystalline precipitate. Filtration and recrystallization further purify the solid, ensuring that next steps in chemical sequences take place without mysterious contaminants. Smaller quantities for academic research often start from succinic acid, though it requires more robust drying to maintain product stability.
Labs depend on succinic dihydrazide most for its behavior as a nucleophile in condensation reactions. Its hydrazide groups react powerfully with carbonyl-containing compounds, leading to the formation of hydrazones or heterocyclic products. Industrial chemists appreciate its use in crosslinking polyvinyl alcohol or polyacrylonitrile, altering the physical characteristics of finished plastics. Other folks put it to work in creating hydrazone-based sensors, which help in environmental monitoring or clinical diagnostics. Synthetic organic chemists often use it for constructing more complex architectures, since attaching two hydrazide termini to a central succinic backbone makes room for more connections and modifications in later steps. With the right catalyst or activating agent, reactions with aldehydes and ketones proceed at a brisk pace under mild temperatures, broadening its reach to a swath of applied research areas.
While the name ‘succinic dihydrazide’ sticks in catalogs, this compound has gone by other aliases depending on context or region. Folks may encounter it as ‘butanedihydrazide,’ ‘1,4-butanedihydrazide,’ or even ‘NSC 19770’ in certain pharmaceutical registries. Many chemical suppliers catalog it under its CAS number 541-73-1 to avoid regional confusion. These alternate names often crop up in patents or older literature, showing just how widespread its use has become from country to country and era to era.
Safety professionals urge proper respect for succinic dihydrazide, since hydrazides sometimes possess unexpected reactivity in the presence of oxidizers. Teams working with this compound keep it away from strong acids and oxidizing agents, since side products can form, and thermal runaway risks emerge in scale-ups. Good practice involves chemical fume hoods and nitrile gloves. Dust masks come in handy during weighing, since inhaling powdered nitrogen-containing compounds can cause lung irritation. Spill cleanup focuses on dilution and collection. On the regulatory side, shipping documents must declare it as a laboratory chemical with proper hazard symbols, though it doesn’t currently sit on major regulatory blacklists. Safety data sheets stress quick face washing if material contacts skin and keeping eyewash stations within reach. Regular updates to workplace training keep teams alert to protocol changes as new research surfaces.
Succinic dihydrazide crops up across quite a few specialty chemical markets. Pharmaceutical researchers rely on its two hydrazide groups to build prodrugs and test new synthetic pathways. Water treatment specialists employ its reactivity when scavenging contaminants or building flocculants. In the world of materials science, its capacity for cross-linking makes it a go-to for modifying polymers in paints and adhesives. Some teams use it for making synthetic fibers more durable, since the resulting materials hold up better in the shake and wash of industrial use. Analytical chemists see value in its ability to trap heavy metals, acting as a ligand in metal chelation and sensor preparation. Even new bioengineering startups have started exploring its use in enzyme immobilization, making processes more efficient in the food and biotech sectors.
Research teams continue to uncover new potential for succinic dihydrazide. Materials scientists in the past decade have blended it with polymers to tune flexibility and resilience for coatings and biomedical devices. Analytical chemists deploy it to develop novel hydrazone probes, detecting trace pollutants or disease markers in blood samples. Drug development pipelines look to its ease of modification, integrating it as a scaffold for more complex pharmacophores. Chemoinformaticians searching databases have noticed a slow but steady uptick in publications linking succinic dihydrazide with enzyme inhibition or antimicrobial screening. In a climate pushing for greener processes, some labs invest in low-waste, solvent-free preparation methods, aiming to deliver the compound with less environmental impact. This pivot aligns with shifting industry priorities as customers demand cleaner, safer chemicals and more transparency in supply chains.
Toxicologists investigating succinic dihydrazide report low acute toxicity in standard in vitro tests, but as with most hydrazides, they watch for breakdown products. Studies show mild mutagenic potential when exposed to extreme conditions that rarely turn up in routine labs. Chronic exposure research remains limited, but agencies recommend using it in closed systems and reducing dust exposure. Animal studies so far point to minimal systemic absorption by oral and dermal routes, but inhalation of fine dusts or reaction-generated fumes does cause respiratory tract irritation in sensitive individuals. Researchers continue to monitor for carcinogenic or teratogenic effects, taking cues from the broader hydrazine category, even though current assessments place it among the less threatening candidates in its class. These ongoing studies keep risk assessments fresh as new regulatory frameworks develop.
Scientific curiosity drives future applications for succinic dihydrazide. More teams plan to push it deeper into green chemistry, reducing solvent use and making it from renewable feedstocks. Biotech innovators see possibilities for attaching the compound to enzymes and proteins, hoping for medical breakthroughs or new types of smart materials. Environmental engineers look to scale its use in water and soil remediation, harnessing it as a chelating agent for stubborn pollutants. Polymer researchers experiment with making biodegradable plastics more processable, balancing strength and flexibility with a lower environmental footprint. Supply chain transparency and sustainability will shape production, as clients increasingly demand traceability and green credentials. With regulatory agencies watching for health and environmental impacts, future producers need to show clear safety documentation and invest in transparent lab and plant practices. The evolving story of succinic dihydrazide reflects both the creativity and caution guiding today’s specialty chemical world.
If you spend any time in a laboratory or talk with folks in the chemical trade, you start recognizing the quiet workhorses—those specialty chemicals clearing the path for research, production, and quality control. Succinic dihydrazide is one of those compounds. It doesn’t make headlines, but it plays a part in a lot of everyday processes, especially when it comes to analytical chemistry and water treatment.
Lab technicians count on succinic dihydrazide mainly for its job as a reagent, especially in water analysis. In colorimetric tests for nitrogen compounds like nitrite and nitrate, succinic dihydrazide proves reliable. By converting nitrite to a readily measurable form, it helps give accurate readings. Municipal water departments and environmental labs benefit from this accuracy, making sure drinking water meets state and federal standards.
Precision isn’t just about making sure the numbers look good on a report. Public health depends on getting these tests right. Nitrate levels, for instance, have a direct link to health issues, especially for babies and pregnant women. By using reliable chemicals in testing, labs ensure communities stay safe. Mistakes in testing don't just waste time—they put real people in danger.
Outside water analysis, succinic dihydrazide shows up in pharmaceutical labs, too. Researchers use it as a building block in synthesizing other molecules, especially hydrazide-containing drugs and polymers. These materials show up in controlled drug delivery or as additives that slow down the degradation of plastics. If you’ve seen work on biodegradable packaging or targeted treatments for diseases, there’s a good chance chemists handled succinic dihydrazide along the way.
Down the road, the demand for smarter, greener chemistry is only going up. Specialty chemicals like succinic dihydrazide let researchers push boundaries—whether they’re designing better water filtration or safer, longer-lasting medicines. The compound’s reliability and the consistency it brings to synthetic pathways make it a quiet ally for innovation.
Handling chemicals always brings up concerns about workplace safety. Succinic dihydrazide carries some risks, so chemical safety protocols can’t be ignored. Protective gloves and goggles, proper ventilation, and attention to disposal all matter. Accidents in the lab or warehouse have ripple effects, sometimes shutting down projects or risking employee health.
There’s room for improvement in public awareness, too. People tend to overlook the behind-the-scenes aspects of water management. Investing in education about laboratory chemicals and their proper use can head off costly mistakes—not just financially but in terms of public trust and health. More transparency from utility companies and regulatory agencies also builds confidence in water quality efforts.
From my own work supporting chemistry educators, I’ve seen that strong fundamentals—good protocols, effective communication, clear safety guidelines—set the stage for chemical applications like succinic dihydrazide to do real good. Supporting ongoing research and bringing new professionals up to speed remain key steps to keeping these important compounds working for everyone’s benefit. Better training and tighter oversight mean fewer errors and more reliable results. Creating partnerships between the chemical industry, universities, and local governments could help bring consistency and transparency, which the field really needs.
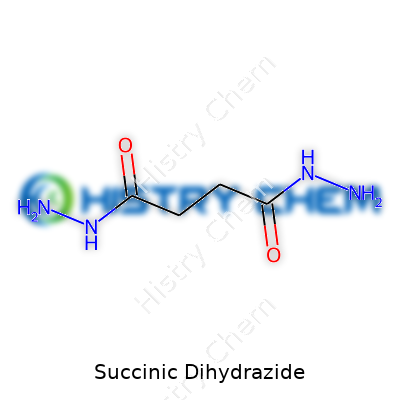
In the chemistry world, every compound carries its own unique identity. Succinic dihydrazide is no exception. With the formula C4H10N4O2, it stands out as a simple and straightforward molecule. The structure comes from succinic acid, swapping both carboxylic acid groups for hydrazide groups. This replacement changes its reactivity, expands how scientists use it in labs, and gives chemists more tools for linking molecules together.
Many people outside the lab aren't familiar with this compound, but its uses pop up quietly in research and manufacturing. Succinic dihydrazide catches the eye of those interested in organic synthesis, especially in pharmaceuticals and special polymers. Its hydrazide groups make it a strong candidate for forming bonds with other compounds. For example, I remember a project where precise linking of molecules meant everything—using a compound like this made that entire experiment possible. The chemical structure gave us flexibility, letting us build larger, more complex molecules without unwanted byproducts.
Handling chemicals, even the less famous ones like succinic dihydrazide, always brings a lesson in respect and caution. Staring down a bottle of white powder doesn't always prepare someone for what it might do if it enters the body or reacts with another chemical. The nitrogen content in this molecule can sometimes worry people since nitrogen-rich compounds often become energetic or even explosive. With C4H10N4O2, you won't run into those issues at room temperature and standard use, but storing it in a dry, cool place prevents scary surprises. In every lab I've worked, clear labels and Material Safety Data Sheets never collect dust for a reason; they get read because safety never goes out of style.
Succinic dihydrazide doesn’t show up in high school textbooks, but it contributes in real ways. Its basic chemical makeup, straightforward formula, and modest physical properties mean it can act as a linking agent or building block. In medicine, researchers chase safer drug delivery systems and controlled-release medicines, and chemicals like this one often appear in early experiments. In industry, the focus leans toward specialty materials where controlled reactivity and custom assembly matter more than mass production.
Science only moves forward when knowledge turns into action. Knowing that C4H10N4O2 represents succinic dihydrazide means more than memorizing another formula. It means understanding a molecule with power to join pieces and spark creativity in chemical design. For anyone in academic labs or the pharmaceutical industry, reliable information about compounds like this one prevents mistakes and sets a good standard for quality. Sometimes, getting a seemingly simple detail right opens up new discoveries. For anyone curious or cautious, a deep dive into the formula and structure offers more than trivia—it delivers practical know-how that supports safe, smart research.
Succinic dihydrazide doesn’t show up in most people’s conversations, but anyone working in a lab, chemical plant, or quality control role knows its significance. With uses in pharmaceuticals and specialty chemicals, this powder often ends up tucked away on shelves for months. Keeping its quality intact is not a set-and-forget job. One wet summer day is all it takes to learn how fast a bottle of fine powder can clump or degrade.
From experience, powders like succinic dihydrazide suck up water from the air quicker than you’d expect. If left open, even for short periods, caking follows. The chemical structure makes it prone to breaking down if exposed to too much moisture. Once this happens, the reagent stops behaving as expected, which can ruin experiments or lead to extra costs in industrial batches. For any researcher or production manager, tossing out an entire order just because the lid wasn’t tight enough feels like burning money. Fact sheets from chemical suppliers like Sigma-Aldrich point out low humidity and tight sealing as top rules—for good reason.
Sticking this reagent on a sunny windowsill or above a radiator might save some shelf space, but waste is almost guaranteed. A lot of breakdown reactions double their speed with every 10°C rise. Labs and factories keep succinic dihydrazide in refrigerators or cool rooms. Keeping things around 4°C to 8°C slows any unwanted reactions to a crawl. Even if you don’t have dedicated cold storage, picking a basement shelf away from heating vents lowers the risk. A thermometer doesn’t lie. It’s easy to lose track of room temperature in bustling spaces, but steady checks stop surprises before they start.
After years behind the bench, one lesson sticks: Clear labeling saves time and headaches. Use a permanent marker with the opening date—especially with chemicals sensitive to age. Transfer a small amount to a working bottle, and keep the bulk stock sealed and untouched. Scooping powder out of the main jar with a damp spatula can turn a whole batch lumpy in a few weeks. Simple habits like gloves or clean tools make a big difference. Sharing space with dozens of other reagents makes mix-ups easy. Getting into a labeling routine helps cut down on errors that only show up during expensive product failures.
Best storage combines a few key ideas: airtight containers, humidity-proof bags, a cool dark cabinet, and scheduled checks. Desiccant packs tossed into the chemical’s box soak up stray moisture. Many labs repurpose old pill bottles with tamper-proof seals for small portions. Suppliers like Thermo Fisher highlight the benefits of opaque, chemical-resistant packaging. For teams handling bulk material in big drums, stick to opening only what you need and resealing with a wrench, not just a twist of the hand. Small details like that keep a valuable powder in top shape.
With rising costs of fine chemicals, paying attention to how succinic dihydrazide spends its time on the shelf just makes sense. Shifting habits to favor cooler, drier conditions with solid labeling and careful handling drives down waste and improves results. Scientists, QA specialists, and production teams know that small steps protect both safety and the bottom line.
Succinic dihydrazide crops up in a range of chemical processes—polymer science, analytical labs, and even in research tied to pharmaceuticals. It comes as a white, crystalline powder. On paper, it might not look like the stuff of danger, but nobody benefits from cutting corners with chemicals of this type. Inhaling dust, getting it on your skin, or mixing it with other reactive substances can trigger problems. Accidents happen fast and tend to leave a mark, so respect for this compound is non-negotiable.
Many years ago, I started my career helping set up undergraduate lab courses. One thing those long hours taught me: even the safest looking powders need the same level of respect as obviously hazardous ones. I remember a time a student ignored gloves, handling a "harmless" compound and broke out in a rash within minutes. A lesson they never forgot. Succinic dihydrazide fits in that category—not a terror in a bottle, yet demanding good habits.
Pulling on a clean lab coat shields you from splashes and stops dust settling on your clothes. Nitrile gloves form a basic defense line for most people. That thin layer saves your skin from direct contact. Protecting your eyes is crucial—a pair of wraparound safety goggles means stray dust in the air never lands on your cornea. A face mask or fine particulate respirator isn't overkill in a poorly ventilated area, especially when weighing out or transferring the powder.
Storage practices matter more than people sometimes realize. Right temperature, away from light and moisture, helps prevent breakdown or accidental reactions with the wrong container. Succinic dihydrazide belongs in a tightly closed, labeled jar, away from direct sunlight and heat. Dryness keeps it from caking and reacting. Proper labeling avoids swap-ups—few lab errors feel worse than using the wrong reagent because of a faded sticker.
Nobody handles chemicals in total isolation. The best labs make safety discussion a regular event. New users benefit from practical training. Old-timers do too, since habits can slip. Quick, simple drills covering spills, burns, or accidental inhalation prepare everyone for the rare but dangerous mistakes. Having the right emergency gear on hand never goes out of style—eyewash stations and chemical spill kits need to be easy to reach and checked often.
Regular review of material safety data sheets helps, too. Updates happen, and relying on five-year-old information gets risky. Management should not only provide MSDS printouts, but set time aside for staff to discuss them. If your team works together to create a culture of caution, accidents stay rare, and everyone keeps their health.
The facts speak for themselves. Direct contact with succinic dihydrazide can irritate the skin, eyes, and airways. Breathing in dust isn’t worth the risk—respiratory protection should become second nature. Good ventilation in storage and handling areas pulls any fugitive dust out of the breathing zone. Controlling clutter on the workbench and keeping containers sealed keeps both spills and accidental mixing off the table.
Thoughtful handling of this chemical wraps around three things: personal protective gear, team habits, and respect for up-to-date chemical information. These simple choices protect your health and everyone around you, every single shift.
Questions about the solubility of chemicals pop up all the time in both classrooms and labs. I remember as a student, water solubility used to be one of those yardsticks for figuring out what you can actually do with a compound. Succinic dihydrazide, used in research and sometimes in specific industrial applications, prompts this question more than you might expect.
Succinic dihydrazide stands out because of its structure—two hydrazide groups attached to a four-carbon backbone. This setup affects how it behaves in different solvents. Water, with its strong hydrogen bonding, loves small molecules that can join in. Think sugars and salts. Succinic dihydrazide, on the other hand, doesn’t join the party as easily. Peer-reviewed resources and hands-on accounts both make it clear: it barely dissolves in water.
Labs trying to use it often have to find workarounds. Encouraging a stubborn powder to mix means relying on heat or using stronger solvents. Even then, many struggle to get more than a trace amount into solution. These headaches matter, because the ability to dissolve dictates how well you can use a compound. Certain tests just don’t run if the sample stays stuck to the bottom of the beaker.
A lot of protocols call for water-based reactions, mostly because water is safe, cheap, and familiar. When you find a low-solubility compound, you might be looking at wasted effort or, worse, misleading results. I’ve watched fellow researchers adjust their plans after long hours chasing clear solutions. It's not just about patience, either. Skip the patience, and you can still end up with inaccurate concentrations.
In pharmaceutical labs, for example, low-water solubility brings real hurdles. Drug developers want their molecules to dissolve, since low solubility often means poor absorption in the body. On the research end, low solubility leads to slow reactions and poor yields. With succinic dihydrazide, some switch over to organic solvents like ethanol or dimethyl sulfoxide. These dissolve the compound more readily, but not everyone likes working with them. Safety, disposal, and cost concerns push many towards water whenever possible.
If you want to improve solubility, one idea involves changing the pH of your solution. This only works for compounds that ionize easily, which isn’t the case for succinic dihydrazide. Grinding to a finer powder sometimes speeds things up, but you’ll never see dramatic changes. Universities often advise trying co-solvents or surfactants—additives that help coax more powder into solution. In practice, this makes sense for experimental work, but rarely scales well.
Outside the lab, people working in production face even tougher choices. Sticking to water means you might need to rethink your entire process. Some companies turn to formulation experts, searching for better carriers or delivery systems. Many professionals accept the limits, sidestepping water altogether and designing methods that suit the compound.
Knowing that succinic dihydrazide doesn’t dissolve much in water shapes the way researchers and manufacturers tackle projects. Decisions about solvents, safety, and equipment all get influenced by solubility data. The next time solubility comes up in discussion, I think about the real work happening on benches and in factories, not just in textbooks.
| Names | |
| Preferred IUPAC name | N'-aminocarbonylhydrazinecarboxamide |
| Other names |
Butanedioic dihydrazide
1,4-Butanedihydrazide Succinic acid dihydrazide Succinohydrazide |
| Pronunciation | /ˈsʌk.sɪn.ɪk daɪ.haɪˈdræz.aɪd/ |
| Identifiers | |
| CAS Number | 541-35-5 |
| Beilstein Reference | 79477 |
| ChEBI | CHEBI:131323 |
| ChEMBL | CHEMBL168922 |
| ChemSpider | 81971 |
| DrugBank | DB03712 |
| ECHA InfoCard | 03b4205a-7fd8-4d60-b851-76f1a93ff2c3 |
| EC Number | EC 206-975-3 |
| Gmelin Reference | 104137 |
| KEGG | C06587 |
| MeSH | D008019 |
| PubChem CID | 85184 |
| RTECS number | VS2275000 |
| UNII | 8T6X5N8G0B |
| UN number | Not classified |
| CompTox Dashboard (EPA) | DTXSID7065169 |
| Properties | |
| Chemical formula | C4H10N4O2 |
| Molar mass | 118.13 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.396 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | -2.2 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 12.35 |
| Basicity (pKb) | 9.26 |
| Magnetic susceptibility (χ) | -57.0e-6 cm³/mol |
| Dipole moment | 4.58 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 373.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -1473 kJ mol-1 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302: Harmful if swallowed. |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | 150°C |
| Autoignition temperature | 215°C |
| Lethal dose or concentration | LD50 (oral, rat): 5000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 > 5000 mg/kg |
| NIOSH | Not Established |
| PEL (Permissible) | PEL (Permissible exposure limit) for Succinic Dihydrazide: Not established |
| REL (Recommended) | 2 mg/m3 |
| Related compounds | |
| Related compounds |
Succinic acid
Succinic anhydride Adipic dihydrazide Glutaric dihydrazide Oxalic dihydrazide |