
Humans have been tinkering and experimenting with chemicals for generations, but phenyl salicylate holds a special place in the history of organic chemistry. First synthesized back in 1887 by Friedrich Wilhelm von Baeyer and Joseph Franchimont, this compound entered laboratories as researchers raced to understand the salicylic acid family. Back then, scientists sought pain relief solutions and looked to modify existing natural compounds for broader uses. Fast forward to today, phenyl salicylate has made its mark not just as a relic of academic curiosity, but as a useful tool in real-world industrial chemistry. People in pharmaceuticals, plastics, and cosmetics all find themselves working with this compound, proving that discoveries from over a century ago can find fresh relevance in modern times.
Phenyl salicylate goes by the alias salol, a name that echoes in both pharmacy textbooks and chemical catalogs. It appears as a white, scaly crystalline powder with a subtle, sweet aroma, sometimes drawing comparisons to the medicinal scents lingering in old-fashioned clinics. This substance dissolves well in alcohol and ether, but holds out when mixed with water, which makes for interesting formulation challenges and possibilities.
You don't need a lab coat to see its unique appearance, but a closer look at its physical traits proves useful for many fields. Phenyl salicylate's melting point hovers around 41–43°C, and chemists appreciate the sharpness with which it transitions from a solid into a liquid. The compound has a molecular formula of C13H10O3, and its molecular weight slides in at about 214.22 g/mol. Salol is stable under ordinary conditions and resists light degradation, but will react if pushed too far with heat or strong acids. Its low solubility in water means it won’t just vanish into a solution—a fact that shapes how it's stored, shipped, and blended in final products. Preparations often need solvents or dispersant strategies to bring out its full potential.
Manufacturers package phenyl salicylate with an eye toward purity and usability. Common grade levels range from 98% purity for regular industrial use, up to 99.5% for pharmacists and fine chemical applications. Labels show off batch numbers, manufacturing and expiry dates, and detailed safety warnings. All the regulatory touchstones appear: CAS Number 118-55-8, proper hazard symbols, health effect statements, and manufacturer contact details. Every container ships with a safety data sheet that talks through first aid, spill responses, and disposal recommendations because, in practice, safety starts with clarity.
The typical industrial route to phenyl salicylate involves a simple yet satisfying bit of esterification. Salicylic acid and phenol come together with the help of phosphorus oxychloride, an acid catalyst favorite among synthetic chemists. The resulting mix cools, and crystals of phenyl salicylate form on their own, ready to be filtered, washed, and dried. The process, straightforward as it is, highlights the craft behind bulk chemical production—one slip on temperature or proportions could lead to wasted batches or poor yields. Some specialty methods play with the reagents or catalysts for better sustainability or lower byproducts, as greener chemistry takes hold. Several major producers keep their tweaks to the process close to the chest, hoping for efficiency or competitive pricing.
Phenyl salicylate isn't just static. In organic syntheses, this ester can break apart under hydrolysis back into phenol and salicylic acid, both invaluable in their own right. It's a convenient source for introducing both fragments into more complicated molecules. When heated or exposed to strong bases, it responds with predictability—a trait highly prized by chemists working to alkylate or otherwise transform its structure. In medicinal design, modifications look to change its pharmacological profile by swapping parts of the molecule, birthing new derivatives that experts hope bring about better activity and reduced side effects. Each modification starts with routine reactions, but often ends in true innovation for pain management or anti-inflammatory treatments.
Walk through any chemical warehouse or scan a catalog, and you’ll find phenyl salicylate listed beside its other names. Salol stands out, but so do benzoic acid, 2-hydroxy-, phenyl ester, and sometimes more arcane titles on old laboratory reports. This can irritate newcomers, but seasoned professionals grow used to the juggling act of synonyms. Some regional datasets, like those upheld in the EU or Japan, enforce standard names for safety and traceability, driving harmonization in global commerce. Products use these names interchangeably, but always circle back to the chemical’s clean structure on certificates and shipping forms.
Using phenyl salicylate in production or research spaces brings with it an obligation to keep health and the environment in mind. Inhaling its dust can trigger mild irritation, and accidental exposure to skin or eyes leads to local discomfort. Long-term studies have yet to reveal any major chronic effects for ordinary industrial users, but prudent practice always wins. Facilities install local exhausts, offer gloves and goggles, and keep eye wash stations handy. In my years overseeing college labs, supervisors never compromised on clear hazard labeling and routine safety briefings. Storage in tight containers, shielded from excess heat or open flames, prevents unnecessary degradation or accidental spills. Violations of these basics can shut down a line or trip up an inspection, making compliance both common sense and good business.
Phenyl salicylate built its legacy on medical applications, once living a double life as both an analgesic and antiseptic in early 20th-century pharmacies. Doctors dispensed it hoping for stomach-soothing effects before more advanced nonsteroidal anti-inflammatory drugs arrived. Its real-world impact grew across plastics manufacturing as an ultraviolet light stabilizer. I’ve seen plastic parts for automotive interiors and consumer goods improved by its inclusion, with colors staying sharp longer in outdoor conditions. In cosmetics, phenyl salicylate finds its way into sunscreens and skin creams where it helps shield sensitive skin from the sun and preserves shelf life for high-end formulations. Some niche uses show up in photographic chemicals and analytical labs, where its ability to undergo neat thermal decomposition gets harnessed for specific tests.
Ongoing research pulls phenyl salicylate into new directions year after year. Chemists push its boundaries with fresh derivatives aiming to balance better pain relief with a reduced risk of side effects or allergic reactions. My conversations with formulation scientists reveal a race to optimize delivery systems through micro-encapsulation, solvent selection, and blending strategies to ensure that phenyl salicylate remains stable but efficient. Environmental chemists look for methods that replace classic production catalysts with more sustainable, less hazardous alternatives—a shift driven by regulatory and consumer pressure. Universities and contract R&D labs frequently publish new findings explaining how modifications can unlock antiviral, antibacterial, or even photoprotective benefits. This constant churn of new knowledge promises new products for both health and industry.
Toxicologists have tested phenyl salicylate thoroughly. Most studies agree on its relatively low acute toxicity, with larger doses causing stomach upset or mild kidney effects, but not the tragic accidents linked to more potent substances. Chronic animal studies suggest the compound doesn’t accumulate and gets excreted fairly efficiently. Regulatory committees still set strict workplace exposure limits—another reminder that in chemical safety, experiences and data must go hand in hand. Long-term skin contact can lead to mild dermatitis, especially in workers without gloves. In my time training young chemists, news about new irritation cases often ended up as teachable moments. The broader toxicology community focuses on metabolic breakdown products and how these interact with organ systems, to avoid surprises down the line as usage patterns shift.
Looking to the years ahead, phenyl salicylate stands to see new life well beyond its role in sunblocks and plastic stabilizers. The search for safer painkillers triggers ongoing molecular modification. Environmental shifts pressure producers to lower byproducts, minimize energy inputs in manufacturing, and switch to renewable starting materials. Advances in material science open doors for phenyl salicylate in photoprotective coatings, next-generation fibers, and even bio-based composites. As more researchers chase multi-functional ingredients, simplicity and versatility could make phenyl salicylate the foundation for everything from advanced packaging to smart pharmaceuticals. Judging by recent patent filings and publicly available research, the classic compound will hold its ground, adding value to industries and sparking interest among newcomers and veterans alike in the chemical business.
If you glance at the ingredient list on some over-the-counter pain relievers or older cold remedies, phenyl salicylate might hide in plain sight. It may not attract attention like acetaminophen or ibuprofen, but it actually plays a multi-faceted role in the world of everyday chemistry. This compound, discovered way back in the late 1800s, sticks around for good reasons that often get overlooked.
I’ve come across phenyl salicylate in a few places during time spent researching pharmaceuticals and dabbling with photography (on film, not digital). At the pharmacy counter, it has cropped up in analgesic balms and powders, mainly because it soothes minor aches and helps with muscle pains. Hospitals once used it more widely as a pain reliever, though its popularity fell off when new drugs arrived.
What kept it relevant over a century later? Its clever chemistry. Not many off-the-shelf compounds act as pain relievers and also keep other ingredients stable. Phenyl salicylate slows down spoilage, helping products avoid that all-too-familiar “off” smell. In sunscreens, its UV-absorbing traits help filter out sunlight, offering a layer of protection for sensitive skin. That’s worth notice now, with sunscreen safety under a harsh spotlight — too many chemicals get by without strong, long-term data on their safety profiles. Phenyl salicylate’s history gives scientists plenty to work with as regulations sharpen up.
In college, a chemistry professor pulled out a dusty vial of phenyl salicylate to explain how esters work in organic reactions. We watched it melt in a warm water bath and crystallize again as it cooled. More than a simple classroom trick, this property makes it useful for demonstrating chemical changes — teachers use it year after year to help students grasp basic science. Good teaching tools stick around for a reason.
Photography enthusiasts may recall it as “salol,” used long ago as a stabilizer for films and photographic paper. It stood up well against heat and humidity, stopping those old photos from disintegrating before they even made it to an album. Nowadays, the industry has moved on to newer compounds, but its example set a bar for stability and reliability.
No commentary is complete without mentioning potential risk. On one hand, phenyl salicylate breaks down into salicylic acid, a relative of aspirin. Regular exposure at high levels could carry some risk for sensitive people, causing irritation or allergic reactions. Product developers need to keep this in mind, ensuring they balance safety with effectiveness. The FDA monitors ingredients like this closely, especially as new research emerges about chemical absorption through the skin or lingering in water systems.
I believe that as consumer safety standards tighten, manufacturers will lean more on real-world historical data, careful lab testing, and transparency about ingredient sources. This means investing in long-term health studies, updating packaging to tell users exactly what’s inside, and listening to public concerns. That’s how trust gets built — not with grandiose promises, but steady, open improvement around compounds like phenyl salicylate.
Phenyl salicylate, sometimes called salol, shows up in a variety of products. You can find it in sunscreens, analgesic ointments, and even some plastics. Chemists first used it over a hundred years ago, and modern manufacturing hasn’t left it behind. Its ability to absorb ultraviolet light turned it into a common ingredient for products that protect skin against sunburn. You might not recognize it on labels, but it plays a role in keeping daily goods functional.
Doctors used to prescribe phenyl salicylate for stomach discomfort. Its structure resembles aspirin, which means it can work as a mild pain reliever and anti-inflammatory agent. As medical research advanced, doctors saw that safer alternatives existed. Medical handbooks don’t recommend it for pain relief or digestive trouble anymore. But you’ll still see it in skincare and as a stabilizer in plastic items, where accidental exposure could happen.
Safety evaluations tell a clear story. In regulated amounts, phenyl salicylate does not create obvious health risks for most people. Extensive animal testing during the 20th century revealed a low risk of toxicity at doses far above what someone would ever get from day-to-day exposure. The U.S. Food and Drug Administration and the European Chemicals Agency both looked at available data and set limits for safe concentrations in consumer products.
That said, phenyl salicylate gets absorbed by the skin and can pass into the body. In rare cases, this substance triggered skin reactions, especially in people who are sensitive to salicylates. Large doses, far more than someone would get from routine contact, could produce symptoms like ringing in the ears, dizziness, or nausea, which parallel side effects seen from aspirin overdoses. Consumer advocates and toxicologists agree: following proper dosage and concentration guidelines matters.
People worry about chemicals building up over time, and for good reason. Research from long-term exposure studies shows that phenyl salicylate doesn’t linger in tissues. The body breaks it into salicylic acid and phenol, which quickly leave through urine. This metabolism pattern supports the idea that short, low-level exposures probably won’t lead to dangerous accumulation.
Environmental health scientists keep tracking any new evidence of harm, especially in vulnerable groups like children or those with broken skin. In rare allergies or high exposures, reactions can happen—this holds true for almost anything, including natural products. Reading ingredient labels and reporting any bad reactions helps public agencies evaluate future risk.
Regulators rely on regular updates from scientists, especially as new research surfaces. Consumers can stick with reputable brands and avoid using products in ways that go against package instructions. If someone knows they react badly to aspirin or have sensitive skin, they might skip sunscreens or ointments containing phenyl salicylate altogether. Health professionals can report unexpected side effects, and both producers and watchdogs now track trends more closely than in the past.
As a parent, I tend to check ingredient lists for anything unfamiliar, especially with products meant for kids. Although the odds of harm stay low, having this awareness gives peace of mind. It doesn’t require uncommon expertise—just a little time in the store and a willingness to ask questions during doctor visits.
Phenyl salicylate shows up in more than a few medicine cabinets and manufacturing setups, used in everything from sunscreens to over-the-counter pain relievers. Its function comes down to soothing pain or protecting skin, but like just about anything else you put on or in your body, there’s the other side—the stuff nobody wants as part of the deal.
The gut tells the story first. Some folks using phenyl salicylate run into nausea or a twisted stomach. Acid reflux can fire up or stick around longer than usual. Throw in a headache or dizziness, and you’re left wondering if trying to feel better just made things worse. This rings even truer for people managing sensitive digestion to begin with—IBS or ulcers, for example, have a way of exposing the downsides of some common chemicals.
The immune system doesn’t always play nice. Skin creams and ointments featuring phenyl salicylate can rub people the wrong way, causing hives, redness, or a rash. Sneezing or breathing troubles sometimes pop up, too. Allergies don’t always give a warning, but they generally show their colors fast, which is why doctors recommend patch testing with new products. In my own extended family, a cousin learned the hard way about a salicylate allergy after a supposedly harmless sunscreen triggered an afternoon of swelling and itching—strong proof that “rare” reactions still matter a great deal to those who have them.
Accidental overdoses don’t just happen with prescription drugs. Too large a dose of phenyl salicylate can tip into toxicity, especially for young children. Signs include ringing in the ears, heavy breathing, confusion, or straight-up collapse. These symptoms mean business and usually need an ER visit. I’ve seen a case up close—a neighbor’s toddler got into a bottle and it turned into a panicked night at the hospital, more than enough proof to keep even “safe” medications under lock.
People with liver or kidney problems might run into trouble more quickly. These organs do most of the chemical cleanup work, but they can’t always handle extra stress from salicylate-based compounds. Doctors flag this risk for good reason; damage usually doesn’t show in plain sight at first, but blood tests tell the story later on. Anyone dealing with organ weakness should always double-check with a healthcare provider before adding new chemicals into their daily routine.
Smart choices and sound rules go a long way. Check the ingredient list and follow label directions—no skipping doses or “just in case” extras. Keep anything resembling medication out of sight and reach for little kids. If you’ve ever had odd reactions to aspirin or similar products, bring it up at doctor visits. People with asthma or chronic hives face more potential triggers, so keeping health records up to date saves headaches in emergencies. The safest path runs through open conversations with a healthcare provider, asking questions without apology, and reporting any side effects clearly.
Most people using phenyl salicylate don’t experience major trouble, but small risks don’t stay small for everyone. Staying aware helps prevent problems before they ever start. Knowledge, plus a dose of caution, keeps the problems off your plate—or out of your medicine cabinet.
Phenyl salicylate has always caught the attention of anyone handling chemicals, whether you’re working in an academic lab, a pharmacy, or an industrial setting. My own experience in the lab taught me early on that proper storage isn’t just about ticking off guidelines—it makes the difference between a safe workspace and a ticking time bomb. Plenty of people overlook this, treating chemicals like benign powders or capsules. Yet mistakes with storage can invite risks you really don’t want on your hands.
Direct sunlight might seem harmless coming through a window, but it can slowly degrade phenyl salicylate. This chemical’s composition doesn’t like UV rays or prolonged heat. I’ve seen bottles left on sunny benches take on a yellowish tint and lose their intended properties, ruining weeks of careful work. The lesson sticks with you: keep containers in a cool, shaded place.
Room temperature works just fine for phenyl salicylate storage—it doesn’t require refrigeration. Stable conditions matter more than extreme cold; unpredictable swings in the environment tend to stress chemicals, leading to clumping, odd odors, or unwanted reactions. In my own lab, a steady 20°C to 25°C keeps the material in good shape month after month.
Once humidity gets into the bottle, phenyl salicylate starts to solidify or forms crusty layers near the cap. Handling becomes difficult, and the chemical loses effectiveness. Silica gel packs inside cabinets or dedicated desiccators act as a cheap insurance policy. These packets suck up stray moisture, keeping the environment as dry as possible. I keep a monthly log to check for soggy gel packs. Changing them on time has saved a few expensive shipments from going to waste.
After each use, the cap should go right back on. Exposure to air over and over again introduces both moisture and dust. I’ve seen people get creative—wrapping the cap with parafilm or even double bagging bottles inside plastic containers just to keep things extra safe. Accurate labeling also avoids mix-ups that nobody wants, especially if the material gets repackaged in smaller quantities for classroom use.
Trained staff can spot a neglected container from a mile off, but students and new lab techs often miss the signs. I still remember mixing up phenyl salicylate with a powder stored in a crumbling cardboard box. I learned, quickly and awkwardly, that standardized containers with tight-fitting lids make all the difference—not just for appearances, but for health, safety, and research results.
Phenyl salicylate isn’t especially volatile or toxic in daily usage, but sloppy storage raises risks that don’t show up right away. Fumes build up, spills creep under benches, and old powder turns less effective. Simple protocols—cool, dry, shaded shelves; tight caps; routine checks; labeled containers—have kept mishaps rare in my own experience. It builds habits across the whole team and gives even newcomers an easy routine to follow.
Chemical safety relies on respect for details and willingness to go the extra mile. Sure, a bottle of phenyl salicylate won’t leap off the shelf and cause chaos on its own. But I’ve seen how human habits—cutting corners and rushing—introduce dangers that would never come up otherwise. Consistent, thoughtful storage isn’t glamorous, but it’s the difference between smooth sailing and a mess nobody saw coming.
Phenyl Salicylate has carved out a small but significant place in both pharmacy and industry circles. Mostly, you’ll find it in products addressing pain, fever, or as an ingredient in sunscreens and antimicrobial mixes. Chemists know it as a substance that relieves mild pain, similar to how aspirin works. Some might know it by the name "salol." The uses range from helping with mild headaches to being a component in some topical creams.
The most common question I come across: How much of this stuff should someone actually take? According to respected medical sources, the adult oral dose usually sits around 300 mg to 1000 mg per day. Folks typically split this into smaller amounts throughout the day. Some older medical formularies suggest taking 150 mg to 300 mg up to three times daily, but it always comes back to what a doctor thinks is safe for you. Weight, age, and what other medications someone takes all affect the answer.
Pediatric use calls for extra caution. There isn't much modern research about dosing for kids, and health professionals tend to steer clear unless there’s a specific and compelling reason. Mothers ask about giving it to their kids for fever. Most clinicians reach for alternatives with more established safety profiles for children, like acetaminophen or ibuprofen.
Taking more than the body can handle brings trouble. Because this compound breaks down into salicylic acid, a cousin of aspirin, the risks are similar—nausea, stomach pain, even ulcers or kidney strain if pushed too hard or taken for long stretches. Folks with certain health conditions such as peptic ulcer disease, bleeding disorders, or aspirin allergy run even higher risks.
Anyone thinking of combining it with other painkillers or anti-inflammatory agents needs to talk to a health professional. Salicylates can stack up in the system, creating a bigger impact than expected. Even folks who tolerate other over-the-counter pain relievers shouldn't assume this one is harmless. Plenty of mild products can get risky without the right starting point.
Mistakes happen when people try to self-medicate for ongoing or severe pain. Phenyl Salicylate, like every pain relief option, serves a purpose but can't fix the root issue in chronic problems. A healthcare provider should weigh in before daily use. For example, most of the time it's better for folks with arthritis to use options that target inflammation more directly.
If you’re dealing with only occasional aches or headaches, and your doctor has said this is fine, keep the dose low and pay attention to how your body feels. Gut discomfort or new symptoms are signals to stop and seek advice.
There's no shortage of pain remedies these days. Acetaminophen and ibuprofen both offer more predictable safety for most people, and healthcare providers often steer patients toward those. Phenyl Salicylate has a role—especially when someone can't use aspirin—but it's not a frontline choice for most patients.
Whenever a person considers any medicine, checking with a professional gives a much better chance at comfort with less risk. Pain and fever act like warning lights. Ignoring the cause while treating the symptom can backfire. In every case, knowledge about how much and how often goes a long way in staying healthy.
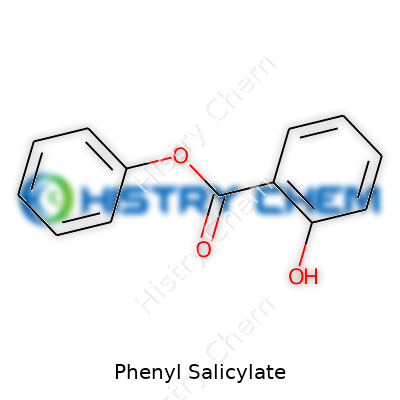
| Names | |
| Preferred IUPAC name | 2-hydroxyphenyl benzoate |
| Other names |
Salol
Phenyl 2-hydroxybenzoate Salicylic acid phenyl ester Benzoic acid, 2-hydroxy-, phenyl ester |
| Pronunciation | /ˈfiː.nɪl səˈlɪ.sɪ.leɪt/ |
| Identifiers | |
| CAS Number | 118-55-8 |
| Beilstein Reference | 1225050 |
| ChEBI | CHEBI:8097 |
| ChEMBL | CHEMBL1419 |
| ChemSpider | 5465 |
| DrugBank | DB13277 |
| ECHA InfoCard | 100.011.349 |
| EC Number | 204-313-9 |
| Gmelin Reference | 82105 |
| KEGG | C02438 |
| MeSH | D010624 |
| PubChem CID | 5055 |
| RTECS number | SN1225000 |
| UNII | 8A5GBT0B8I |
| UN number | UN2811 |
| Properties | |
| Chemical formula | C13H10O3 |
| Molar mass | 214.22 g/mol |
| Appearance | white crystalline powder |
| Odor | Odorless |
| Density | 1.174 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | 3.9 |
| Vapor pressure | 0.001 mmHg (25°C) |
| Acidity (pKa) | 13.6 |
| Basicity (pKb) | 13.38 |
| Magnetic susceptibility (χ) | -75.5 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.595 |
| Viscosity | 3 cP (25°C) |
| Dipole moment | 2.61 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 308.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -97.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -6354 kJ/mol |
| Pharmacology | |
| ATC code | N02BG06 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin and serious eye irritation. May cause respiratory irritation. May cause allergic skin reaction. |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS06,GHS07 |
| Signal word | Warning |
| Hazard statements | H302: Harmful if swallowed. H319: Causes serious eye irritation. H335: May cause respiratory irritation. |
| Precautionary statements | P210, P261, P264, P271, P280, P301+P312, P304+P340, P312, P330, P403+P233, P405, P501 |
| Flash point | 78 °C |
| Autoignition temperature | 540°C |
| Lethal dose or concentration | LD50 oral rat 1018 mg/kg |
| LD50 (median dose) | LD50 (median dose): 1017 mg/kg (oral, rat) |
| NIOSH | JN8575000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 3 mg/kg |
| Related compounds | |
| Related compounds |
Salol
Salicylic acid Phenol Aspirin Methyl salicylate Benzyl salicylate Acetaminophen |