
Monoethyl adipate didn’t spring from thin air; chemists in the twentieth century nudged and tinkered with adipic acid and its derivatives, aiming to harness manageable, versatile esters for plasticizers, lubricants, and solvents. Driven by the surging plastics and coatings markets after World War II, research labs pushed beyond standard phthalates to solve challenges in flexibility and volatility. Ethyl and other short-chain monoesters promised new combinations of solvency and plasticization that became hard to ignore as stricter health guidelines landed on desks. Over time, monoethyl adipate slipped quietly into more formulas, humming behind the scenes in factories and pilot plants.
Monoethyl adipate forms by reacting adipic acid with ethanol, swapping one hydrogen for an ethyl chain. The result: an oily, slightly viscous liquid that doesn’t shout for attention. Industrial producers bottle it from purification columns, checking for clarity and odorlessness before shipping drums out for coatings, inks, cosmetics, and plastics. Chemists working with this ester appreciate its moderate evaporation rate; it settles comfortably between harsher solvents and heavier plasticizers, giving product designers finer control over softness, texture, and stability without driving up costs.
Monoethyl adipate brings a molecular weight of about 188.21 g/mol to the table, with mild polarity thanks to its ester and carboxyl groups. It pours easily at room temperature, remaining liquid down to around -30°C and resisting boiling until over 290°C. Unlike the more common diethyl or dimethyl adipates, the monoethyl version keeps a free carboxyl available, which ramps up solubility in water and allows easy mixing with alcohols, glycols, and some hydrocarbons. Its moderate viscosity allows it to spread and penetrate surfaces. It’s these physical quirks that make it a quiet favorite in specialty coatings and personal care emulsions.
Suppliers stake their reputations on technical sheets, listing guaranteed purity—usually north of 98%—as well as water content, color (APHA below 50), acidity, and specific gravity. Labels must flag flammability, storage requirements, and appropriate hazard codes. Anyone handling monoethyl adipate checks labels for batch codes and production dates, since hydrolysis or contamination can trigger instability. Safety Data Sheets (SDS) don’t sugarcoat caution: those free carboxyl groups react under heat or with bases, and organic esters can irritate the skin or eyes if splashed. Storing in steel or HDPE drums, out of sunlight and away from strong alkalis, beats facing a spoiled batch or dangerous pressure build-up down the line.
Making monoethyl adipate isn’t just schoolroom chemistry, but the backbone remains the Fisher esterification reaction. Scientists or plant operators combine adipic acid with excess ethanol in a reactor, add sulfuric acid as catalyst, and heat the mix. Water forms as a byproduct, so operators rig up columns or nitrogen purges to draw it off, driving the reaction forward. Once neutralized and washed, distillation pulls off excess ethanol and concentrates the product. Quality control hinges on precise temperature holds, solvent removal, and careful catalyst quenching; even a small slip leaves behind diester or unreacted acid, muddying up downstream applications.
Monoethyl adipate never stays idle in a flask. Chemists often use that accessible carboxyl group to build bigger or more functional molecules. Amidation with amines crafts surfactants and specialty lubricants. Further esterification yields diesters for flexible polyvinyl chloride, breaking down stiffness without introducing unwelcome phthalates. Heat or strong acids can push hydrolysis, reverting the molecule to ethyl alcohol and adipic acid—useful for recycling or cleaning up spills. Under the right currents and reagents, monoethyl adipate tags along as a plasticizer in polymers or a dispersing agent in pigment preps, proving its worth well outside simple formulas.
Trade and lab shelves can mask monoethyl adipate under a variety of names: Adipic acid monoethyl ester, Ethyl hydrogen adipate, and Monoethyl hexanedioate pop up in catalogs. Chemists recognize these synonyms quickly due to their repetitive use in method papers, lab guides, and technical bulletins from companies mixing plasticizers or prepping test solutions. Regulatory filings rely on its CAS number (626-86-8), allowing customs inspectors, environmental officers, and chemists to speak the same language while reeling off safety checks or usage declarations.
Factories sticking with monoethyl adipate must lean on robust hazard assessments. Regular air monitoring, spill kits, fire extinguishers, and personal protection block headaches before they start. Ventilation and tight seals keep inhalation risks in check, as hot vapors can sting noses and throats. Gloves and goggles guard from accidental splashes. Drums shouldn’t pile too high, and all containers need clear hazard diamonds. Waste streams send hydrolyzed residues for incineration or authorized chemical treatment, not down the drain. Staff follow established guidelines—OSHA, REACH, or their local equivalents—to document usage, exposure, and proper disposal, aiming for compliance but above all for workplace health.
Factories don’t just keep monoethyl adipate in the closet for emergencies. It slips into formulas for dyes, coatings, and plastic intermediates, thanks to easy process adjustments. Paint chemists use it for fine-tuning gloss and flow, while ink makers rely on its volatility for fast drying but reduced harshness. In cosmetics, its moderate solubility and dispersion help creams blend smoothly or lotions stay stable. Lubricant engineers slip a dose into synthetic esters for friction modifiers, adding durability to machinery in challenging heat or cold. Environmental scientists sometimes turn to it as a safer alternative to traditional phthalate plasticizers, especially since monoesters break down more predictably in soil and water.
Monoethyl adipate’s versatility attracts a steady stream of research projects. Universities and private labs probe its behavior as a base molecule for biodegradable polymers, hoping to develop packaging with the durability of traditional plastics but easier compostability. Some papers highlight how the free carboxyl affects polymerization, opening doors for purpose-built adhesives and elastomers. Research also follows new catalysts and green chemistry routes, experimenting with less hazardous acids or enzyme systems. Chemists document migration rates in food packaging and toys, seeking to confirm monoethyl adipate’s reputation as a cleaner, lower-toxicity plasticizer. New patents emerge on blending it with other esters for tailored mechanical properties.
Toxicologists study monoethyl adipate’s impact with a wary eye after decades of concern about plasticizers. Animal inhalation studies suggest irritation at high doses, but most regulatory assessments point to lower acute and chronic toxicity than phthalate diesters. It doesn’t linger or accumulate in fat, breaking down quickly in the body and the environment. Still, data sets remain limited on developmental and reproductive outcomes, so health agencies restrict its use in direct-contact food products until further notice. Workers handling large volumes report mostly mild irritant effects similar to other short-chain esters. Research groups keep chasing long-term studies on metabolites and breakdown pathways, responding to stricter European and North American chemical directives.
Market analysts and research teams keep scanning for better plasticizers and intermediates as regulations and customer preferences shift. Monoethyl adipate stands out as a compromise: not the cheapest or most powerful plasticizer, but one that offers a balance of safety, biodegradability, and processability that appeals to product developers facing mounting restrictions on traditional options. More suppliers invest in greener synthesis methods and circular economy approaches, using waste alcohols or renewable adipic acid routes. These changes point to a future where monoethyl adipate features in more sustainable packaging, coatings, lubricants, and personal care bases, as the rush for safer chemicals shapes manufacturing decisions across the globe.
Monoethyl adipate gets plenty of attention in industries that rely on plastics, coatings, and personal care products. Its main job is to act as a plasticizer, which means it helps make plastic materials more flexible and softer. Without plasticizers, some materials feel rigid and even brittle, which leads to cracks and early breakage. I’ve seen this play out in old toys or cables from decades back. When a flexible material ages poorly, odds are it didn’t benefit from a good plasticizer.
Monoethyl adipate blends into the polymer matrix and lets the chains of plastic move more easily. You’ll find it in coatings for wires, flexible films used in packaging, and sometimes in adhesives that need both strength and a little give. In the cosmetics world, this compound gets used because it helps spread creams and lotions more smoothly, and helps active ingredients dissolve evenly. That silky feel in a moisturizer or sunscreen can owe a lot to chemicals like monoethyl adipate.
Safety questions pop up with many chemicals in manufacturing. Monoethyl adipate doesn’t show the same level of toxicity as some harsh phthalates, which has helped its reputation. The U.S. Environmental Protection Agency reviews substances like this one for risks to people and the environment. Short-term studies don’t turn up strong red flags for skin irritation or other obvious side effects in most people. Still, it pays to remember that every chemical carries risks if handled wrong or misused—a lifetime in the lab taught me not to take shortcuts or ignore proper ventilation.
Waste and the environment matter too. As older plasticizers get phased out over health or pollution concerns, the door opens for safer alternatives. But tracking what happens when monoethyl adipate enters water or soil after use still deserves more attention. The push for “greener” chemistry encourages more people in the chemical industry to ask questions about life after disposal—where do these molecules eventually end up, and how do they behave? Some evidence suggests monoethyl adipate biodegrades more easily than stubborn phthalates, but monitoring continues.
Companies keep searching for ingredients that balance cost, performance, and safety. Monoethyl adipate hasn’t solved every problem, but it offers manufacturers another tool when phthalates or other plasticizers fall short or attract regulation. In day-to-day work, anyone using this chemical should keep an eye on its source and purity—shady sources sometimes cut corners on safety. Reliable suppliers provide testing data and traceability. When regulations evolve, businesses can avoid headaches if they maintain solid documentation and good manufacturing practices.
Better chemical design relies on frank discussion between researchers, manufacturers, and regulators. Chemists stay under pressure to develop compounds that aren’t just cheap and useful, but also less likely to harm people or the planet. Real improvement happens when experts share findings and push for safer options with open eyes. Having spent years in industry labs, I know that taking shortcuts just circles back as bigger problems down the road. If we keep that lesson front-and-center, compounds like monoethyl adipate can earn a place in safer, cleaner manufacturing.
Monoethyl adipate turns up in a pile of everyday products, including plastics, cosmetics, and even cleaning items. You probably won’t spot it on the label at the neighborhood store, but manufacturers know this chemical for its flexibility and ability to soften materials. Before a chemical like this lands in household goods, it goes through safety checks. Still, seeing a long name on an ingredient list can spark some worry.
Scientific testing on monoethyl adipate has focused on its ability to irritate skin or trigger an allergic reaction. Research points to a low risk of causing major issues for most people, but some might notice mild skin irritation with repeated, concentrated exposure. The database from the European Chemicals Agency places monoethyl adipate in the “low concern” group for toxicity. It has a high threshold for skin and eye irritation, though no chemical is completely risk-free. Proper handling and formulation protect the end user from totals that could really do harm, according to regulatory data.
You’re most likely to meet this chemical in a lotion, sunscreen, or cleaner — not directly from a lab bottle. Regulations make companies check concentrations, and products usually carry levels set well below danger zones. The U.S. Environmental Protection Agency and the Food and Drug Administration both set up guidelines for safe exposure. At home, nobody uses raw monoethyl adipate. It’s mixed in low concentrations, and washing skin with water after use further shrinks any risk.
Talking to people with sensitive skin, some recall redness or itching after repeated exposure to new cleaners or creams. These cases rarely point straight to monoethyl adipate, though, since many products blend heaps of chemicals. People working in factories might see higher concentrations in raw form, so wearing gloves and proper safety gear makes sense.
Animal testing at extremely high doses showed minor irritation but didn’t cause long-term harm. Regulatory groups keep an eye on new data. They update limits if anything shifts in scientific understanding. As of now, scientific panels have not flagged it as a cancer risk, reproductive toxin, or hormone disruptor.
Cosmetic and cleaning companies stand to gain consumer trust by sharing ingredients and concentrations. Many already do, thanks to changing demand and tougher rules around labeling. Trust goes up when companies say exactly how much of each chemical sits in the bottle. Dermatologists recommend patch testing new products, especially for those with allergies or eczema. Organizations like the Environmental Working Group give ratings on chemical safety, helping people make informed choices.
Reviewing labels matters. For families with children or folks with delicate skin, picking unscented or “hypoallergenic” products often helps. Industry watchdogs should keep pushing companies to reconsider any ingredient raising eyebrows with new research. Government and independent groups must stay funded for ongoing studies to catch long-term risks and keep safety standards honest. Good science means going back to the drawing board if evidence shifts, not waiting for problems to pile up.
Real life means coming into contact with unfamiliar chemicals every day. Monoethyl adipate shows a clean record so far, but smart choices and regular safety reviews matter for everyone’s health. People deserve clear information and should never feel left in the dark about what touches their skin.
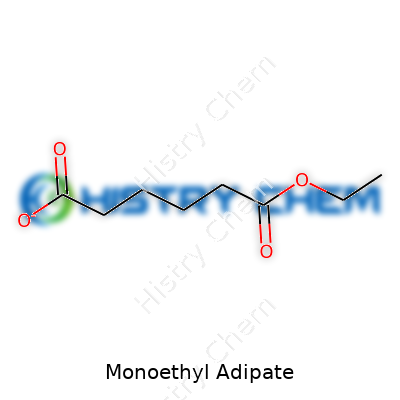
Monoethyl adipate is a chemical name you don’t hear tossed around at most kitchen tables, but it sits behind the scenes in more places than you might think. Its chemical formula is C8H14O4, and it comes from combining adipic acid with ethanol. This forms an ester – the result of an acid and an alcohol locking together and dropping water as a by-product. You get one ethyl group stuck to one side of the adipic acid chain, which explains the “monoethyl” part.
The structure of monoethyl adipate gives it certain properties that help in industry and research. The real hook with this molecule is in plastics, coatings, and sometimes as a special solvent. In my previous work around paint and plastic testing labs, I learned how much hinges on the performance of such compounds. You think you’re buying something simple, like a paint or a flexible bottle, but chemists have spent ages searching for the right blend of flexibility, durability, and safety.
C8H14O4 doesn’t just pop up by itself. It comes from partially reacting adipic acid (C6H10O4) with ethanol (C2H5OH), forming one ester bond. Lab folks appreciate how easily it blends and dissolves other substances. If you’re looking to make coatings that need to spread evenly but dry without chalky residue, these esters come in handy. Monoethyl adipate has also been looked at as a sort of intermediate; it can get turned into other useful chemicals or polymers.
Experience in product safety shows that formula details are not just trivia. Chemical formulas like C8H14O4 are tracked in big safety databases. People who work in chemical plants have to pay attention to handling and storage instructions. Spills or accidental releases bring regulatory eyes. Monoethyl adipate doesn’t ring major alarm bells in toxicity compared with big industrial risks, but its safe handling still requires common sense — gloves, goggles, and decent ventilation matter in any lab or production line.
Disposal creates another challenge. Small molecules get taken up by water and soil systems, so regulators want to see proof that monoethyl adipate breaks down safely. Industry researchers test for biodegradability and the chance for lingering by-products. In this field, transparency about the chemical formula helps watchdogs and companies keep products in check. Nobody wants another headline about hidden environmental hazards.
With so many different chemicals in circulation, learning more about their building blocks pays off. More companies use digital tracking for chemicals, attaching full ingredient lists to finished products. Labeling with clear chemical formulas like C8H14O4 helps not just regulators, but customers and end-users. Teachers can draw on real-life examples of molecules like monoethyl adipate to get students thinking about how molecular tweaks drive big changes in everyday objects.
Openness also builds trust. Sharing formulas, plus toxicity and breakdown profiles, gives people a way to judge risks for themselves. By investing in better lab testing and more data sharing, both manufacturers and watchdogs can pull troublemakers from the supply chain before they hit a landfill or recycling center. With more eyes on the formulas and their impacts, the risk drops – and the benefits just keep stacking up.
Monoethyl Adipate, often recognized as a solvent or plasticizer, comes into play across paints, coatings, and specialty chemical production. It’s not as volatile or hazardous as several industrial solvents, but careless storage or sloppy handling can still mean trouble. I’ve seen folks cut corners with chemicals considered “benign,” and the aftermath speaks volumes: product contamination, damaged containers, and health concerns for workers, all of which create job site headaches and unnecessary costs.
Issues start stacking up fast in facilities where storage habits slip. Monoethyl Adipate should go in a cool, dry spot. High temperatures cause degradation, and humid or damp spaces may encourage rust on steel drums or the growth of mold if a spill isn’t cleaned thoroughly. Direct sunlight will accelerate breakdown and could mess with the product’s strength, which no one wants hitting their end product.
I’ve learned it’s smart practice to keep containers off the floor, using pallets or shelving. This blocks moisture from creeping up from concrete, and in flood risks or high-humidity regions, that barrier really pays off. It also keeps you compliant with worker safety guidelines, which supervisors and inspectors both appreciate.
Storing Monoethyl Adipate in high-density polyethylene drums or tanks creates fewer headaches down the road. Steel containers work as long as there’s adequate lining—bare steel rusts, and contamination in the finished product eats into profit. Tight seals matter, since loose or leaky lids mean evaporative loss and environmental complaints piling up. I’ve seen a simple loose cap cost a shop several gallons over just a few days, making something as minor as a gasket check worth the couple minutes it takes.
Proper labeling isn’t just red tape. Having clear, legible information on every drum means no confusion when workers go to grab product. Mixing up chemicals because of faded or missing labels has caused some disasters. Once you watch someone pour a solvent into the wrong line—destroying a whole batch—you realize why every storage area needs checks for readable, up-to-date labels. Flammable materials should stay far from heat sources, open electrical panels, and busy forklift routes to cut the risk of ignition if a spill happens.
Skin exposure or splashes with Monoethyl Adipate might not be acutely toxic, but it’s smart to use chemical-resistant gloves and safety glasses. In case of a spill, a quick rinse with water usually does the trick, but for bigger messes, absorbent pads and proper disposal procedures save time and potential citations. Keeping a spill kit handy isn’t overkill—it's a sign of a responsible operation.
Ventilation goes hand in hand with safety, even if the vapors aren’t overwhelming. Fresh air stops fumes from building up, protects workers, and helps a shop stay well below occupational exposure limits. Teams that work with solvents daily benefit from regular reminders on how to safely handle drums, open caps, and transfer liquids using pumps instead of just tipping containers by hand. These small changes prevent accidents that often come from rushing through basic tasks or skipping steps.
Regular spot checks make all the difference, something I’ve witnessed through countless safety walkthroughs. Catching dents in drums, corroded fittings, or sticky residue early means less mess and lower risk all around. Documenting these activities can feel tedious, but in an inspection or an emergency, complete records speak louder than excuses.
Staying ahead of storage and handling doesn’t need a huge budget or fancy upgrades. Leadership investment in basic safety training and clear organization shows up in productivity and worker health. Monoethyl Adipate may not spark headlines with dramatic accidents, but small lapses can chip away at a business over time. Paying attention to the basics—cool, dry storage, sealed and labeled drums, proper gear, and maintenance—keeps operations running smooth and safe without drama.
Monoethyl Adipate doesn’t pop up on store shelves. If you’re searching for it, odds are high you work in a lab or handle specialty manufacturing. Chemicals like this serve as plasticizers, solvents, and sometimes intermediates during synthesis for polymers and coatings. There’s a lot of pressure to get the right grade and ensure the source can verify both quality and legality. I’ve worked with specialty labs that struggled to find specific intermediates, and frustration piles up quickly when distributors dodge your emails or only offer bulk truckloads.
Distributors who deal with laboratory or industrial chemicals usually stock Monoethyl Adipate. Sigma-Aldrich, TCI America, Thermo Fisher Scientific, and Alfa Aesar typically cover both academic and industrial clients. They can ship small quantities for research or scale up to drums for manufacturing. Sometimes you have to look further afield; international suppliers like Tokyo Chemical Industry (TCI) or India-based Central Drug House (CDH) open up more options, especially if domestic stocks run low.
Most suppliers require proof the buyer is part of a legitimate business or research institution. I remember ordering rare reagents for a college lab, where we couldn’t even get a quote without providing a university account and a signed safety sheet. If you’re ordering as an individual, expect vendors to say no. This reduces risk of misuse and lines up with chemical safety regulations. In my experience, staffers at reputable chemical suppliers will share Certificates of Analysis and Safety Data Sheets up front—just ask. If someone refuses, walk away.
Search for registration numbers and proper contact info. A real distributor won’t hesitate to give their full business address and tax identification. Call the number, not just email—phony sites lift catalogs straight from trusted brands. Google reviews, trade group memberships, and LinkedIn listings tell a lot. I saw labs get scammed by a website that looked convincing, but a quick check showed the address belonged to an empty lot. A real supplier wants long-term customers, so expect transparency and paperwork at every step.
Monoethyl Adipate can end up in consumer goods or high-purity lab experiments, so purity, traceability, and environmental handling make a real difference. Chemical accidents and unsafe storage have cost lives and millions in damages. I’ve seen friends spend weeks cleaning up after a single mislabeled drum. Sources with ISO certifications or strong sustainability plans help protect people and planet. It pays to check if the supplier describes their waste management or sourcing standards. If not, that’s a red flag.
Prices bounce around based on grade and volume. Expect to fill out paperwork for shipping, customs, and product safety. Some reagents need refrigerated or special transit. International orders might sit in customs if forms get messed up—double check everything. Most buyers end up calling ahead to ask about lead times and delivery windows, especially if you need product fast for a deadline. Good suppliers will walk you through the forms directly. If you’re uncertain at any point, it saves time to request help before cash leaves your account.
| Names | |
| Preferred IUPAC name | Ethyl hexanedioate |
| Other names |
Ethyl adipate
Adipic acid monoethyl ester Monoethyl hexanedioate Ethylic acid adipic monoester Ethyl hydrogen adipate |
| Pronunciation | /ˌmɒn.oʊˈɛθ.ɪl ˈæd.ɪ.peɪt/ |
| Identifiers | |
| CAS Number | 626-86-8 |
| Beilstein Reference | 1082392 |
| ChEBI | CHEBI:88544 |
| ChEMBL | CHEMBL21737 |
| ChemSpider | 10921 |
| DrugBank | DB14096 |
| ECHA InfoCard | 03e6d4b9-0eff-491e-ac86-dd8196d3b241 |
| EC Number | 203-289-7 |
| Gmelin Reference | 8015 |
| KEGG | C19688 |
| MeSH | D000733 |
| PubChem CID | 8579 |
| RTECS number | AU8400000 |
| UNII | F41E6UC31M |
| UN number | UN No.: "UN2527 |
| Properties | |
| Chemical formula | C8H14O4 |
| Molar mass | 202.23 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Mild odor |
| Density | 1.042 g/cm³ |
| Solubility in water | Soluble |
| log P | 0.72 |
| Vapor pressure | 0.00048 mmHg (25°C) |
| Acidity (pKa) | pKa ≈ 4.4 |
| Basicity (pKb) | 10.75 |
| Magnetic susceptibility (χ) | -6.41×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.435 |
| Viscosity | Viscosity: 7.5 mPa·s (25 °C) |
| Dipole moment | 2.54 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 290.4 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -726.2 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3707.1 kJ/mol |
| Pharmacology | |
| ATC code | Not assigned |
| Hazards | |
| Main hazards | Causes serious eye irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | P264, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-1-0-N |
| Flash point | Flash point: 213 °C |
| Autoignition temperature | 420 °C |
| Lethal dose or concentration | LD50 (oral, rat): 8,000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat 7,500 mg/kg |
| NIOSH | NA0490000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Monoethyl Adipate: Not established |
| REL (Recommended) | 50.00 % |
| Related compounds | |
| Related compounds |
Adipic acid
Diethyl adipate Dimethyl adipate Adipoyl chloride |