
Hexadecanedioic acid, sometimes popping up as C16 dicarboxylic acid, traces its roots to the intense search for robust, versatile molecules that could feed the rising needs of twentieth-century polymer science. As chemists hunted for ways to improve synthetic fibers and plastics, this long-chain dicarboxylic acid found favor for its ability to deliver flexibility and durability. Modern-day labs, influenced by pioneers in organic synthesis, continue to appreciate hexadecanedioic acid’s backbone as they design specialty nylons or experiment with biodegradable polymers. Whether spilled from early chemical reaction flasks or engineered by modern biotech, hexadecanedioic acid has traveled from purely academic interest to staple reagent status in dozens of fields.
Hexadecanedioic acid stands out in the family of saturated dicarboxylic acids. You’ll find it as white odorless powder or crystals, and the substance holds great value for chemical engineers, lab researchers, and production managers in plastic, cosmetic, and pharmaceutical industries. Thanks to a robust molecular structure, it serves as a practical choice for polymer chain extension and modification. Buyers look for consistency, high purity (often above 98%), and minimal trace contaminants—especially metals and other carboxylic acids that can interfere downstream. Product codes or catalog references from suppliers often use names like Hexadecanedioic acid, 1,16-Hexadecanedioic acid, or hexadecane-1,16-dioic acid—so chemical buyers need to pay careful attention.
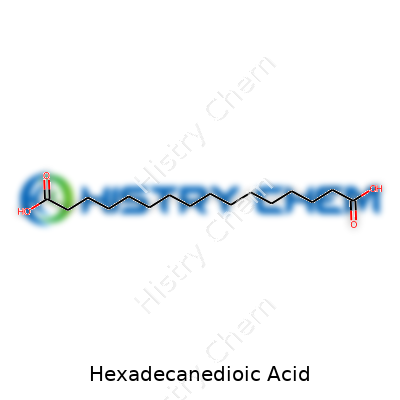
This organic acid features a chain of sixteen carbon atoms, topped at each end by a carboxyl group (-COOH). Hexadecanedioic acid weighs in at about 286.41 g/mol, and the structure (HOOC-(CH2)14-COOH) describes a straight path ideal for linking into larger molecules. Melting begins around 124–127°C, and the material resists dissolving in water. It finds better solubility in organic solvents like ethanol, methanol, or acetone. Chemical reactivity centers on those carboxyl ends, which proves valuable for building esters, amides, or polyester backbones. The acid earns a solid place on bench shelves for reliability, predictable melting, and clean transformation in standard organic reactions.
Laboratories demand a clear snapshot of purity and contaminant profile, so suppliers run hexadecanedioic acid through methods like HPLC, GC-MS, or simple melting point tests. Labels reflect batch number, net mass, manufacturer, molecular formula (C16H30O4), and hazard symbols. Storage conditions get noted—usually a cool, dry place away from oxidizing agents. Technical datasheets call out heavy metals (below 10 ppm), moisture content (often under 0.5%), and ash ranges to ensure end-users don’t encounter bottlenecks mid-project. Custom orders for research sometimes require certificate of analysis that details even finer points, like optical clarity or presence of color bodies.
The journey from simple molecules to hexadecanedioic acid includes both classic chemical synthesis and modern enzymatic or fermentation pathways. Traditional methods, such as oxidative cleavage of long-chain alkenes or fatty acids, dominate industrial production. Some plants use permanganate oxidation on hexadecene or work with ozonolysis, followed by careful purification. Greener approaches, which make heavy use of engineered microorganisms, have started picking up in biorefineries. Modified yeast or bacteria digest plant oils and secrete saturated dicarboxylic acids. These methods reduce reliance on harsh chemicals, trimming both emissions and hazardous waste. Both chemical and biological approaches race to lower production cost and maximize throughput, but greener fermentation methods now attract renewed attention as markets look for sustainable feedstocks.
Hexadecanedioic acid’s functionality centers on two reactive carboxyl groups. Synthetic chemists often convert the acid into esters or amides via reaction with alcohols and amines, sometimes hinting at nylon-16,16 production or customized surfactants. Under dehydrating agents or condensing catalysts, the molecule links into larger chains—imagine a sturdy polyester backbone or a hydrolytically stable lubricant base. The straight saturated chain resists unwanted side reactions, making the acid a steady performer in polymerization. Oxidative or reductive tweaks can also produce unusual intermediates for advanced research. I’ve seen labs push for side-chain modifications using halogenation, hydrogenation, or coupling with larger aromatic groups; this expands utility into fields like specialty coatings or even medicinal chemistry.
Chemicals like hexadecanedioic acid sneak into inventory under a string of aliases. You’ll notice alternative names like palmitic acid dicarboxylic acid, 1,16-hexadecanedioic acid, or hexadecane-1,16-dioic acid written on drums and containers. Older suppliers might shorten it to C16 diacid. Flipping through supplier catalogs, you sometimes spot variations from different language markets, or unique catalog numbers for pharmaceutical or industrial grades. Researchers keeping up with the literature should note these synonyms, as missing a single variant can scatter search results across journals or patent filings.
Handling hexadecanedioic acid resembles best practices for most fine organic powders. Direct skin or eye contact can cause light irritation, and inhalation of dust never does anyone favors. Safety data sheets recommend working with gloves, goggles, and dust mask—at a minimum. In my lab days, we kept the material in tightly sealed drums and made sure to sweep up spills with care to avoid airborne particles. Storage away from oxidizing chemicals helps minimize risk. Disposal follows local hazardous waste guidelines, although the acid itself poses low long-term threats outside of acute exposure zones. Newer handling protocols include automated powder dispensing to cut down on spills and worker exposure.
Synthetic fibers rank high on the list of uses for hexadecanedioic acid. Nylon-16,16 and other specialty polyamides pick up advantages in strength, chemical resistance, and resilience. Lubricant manufacturers blend it into synthetic greases that need to function at high temperatures or under tough mechanical loads. Cosmetic companies look at it as a precursor for emollients, skin-conditioning agents, and texturizing ingredients. Recent advances in biodegradable plastics have also opened doors for this acid as a way to extend molecular chains or improve hydrolysis resistance in green packaging. In medical fields, surface coatings derived from this molecule pop up in stent production and drug-delivery polymers. It’s clear users in every sector want reliable supply, clean specs, and consistent reaction profiles.
R&D teams pay close attention to both the synthetic routes and the applications pipeline for hexadecanedioic acid. Scientists aim at lowering production costs and limiting environmental load—fermentation and enzyme-mediated synthesis see heavy investment. Polymer science research stretches into custom chain lengths and functionalized versions that target next-generation plastics or biomedical devices. University labs hunt for tailored modification methods, sometimes cross-linking with new monomers or exploring blends for enhanced mechanical properties. Industrial chemists look for greener catalysts and closed-loop production cycles that capture emissions and recycle waste. Teams also keep an eye on REACH and green chemistry compliance, to stay ahead of changing regulatory standards.
Toxicological profiles of hexadecanedioic acid rank moderate compared to smaller-chain carboxylic acids. Acute oral toxicity studies in rodents set high LD50 thresholds, meaning substantial exposure doesn’t trigger widespread danger. Skin irritation stands out as the main occupational hazard, with direct and repeated exposure at powdered form occasionally producing dermatitis. Environmental release appears limited since most production runs in closed loop, but researchers keep tabs on bioaccumulation and breakdown products to avoid surprises. Regulatory bodies look for chronic exposure data; the acid shows full mineralization in soil microbiota studies, which lowers long-term ecological risk. Pharmaco-toxicologists test metabolic fates in preparation for pharmaceutical use, since metabolic clearance and lack of interference with critical enzymes drive clinical adoption.
Growth in sustainable materials manufacturing promises a busy decade ahead for hexadecanedioic acid. The push for green plastics, safer textile finishes, and biobased lubricants presents big commercial opportunity. Researchers expect new routes via engineered yeast and bacterial strains to cut costs and increase product purity. Regulatory pressure forces factories to slash emissions—so any breakthrough that lets producers recycle solvents or waste heat will see quick adoption. Specialty applications in medicine seem fast-tracked as drug delivery and implantable device manufacturers search for new materials with proven safety profiles. Customers banking on the next generation of sustainable packaging or automotive parts need stable, affordable sources of high-purity C16 diacid. Sticking with green chemistry, stepping up analytics, and keeping quality standards high will set apart the next round of industry leaders.
Hexadecanedioic acid sounds like something you’d only see in a chemistry textbook, but its uses are much closer to everyday life than most realize. This is a dicarboxylic acid, which means it has two carboxyl groups. Chemically, that structure gives it special features that companies can put to work in everything from tough plastics to skin creams. Anyone who’s touched a plastic handle or bought certain cosmetics has probably run into products that depend on this ingredient working behind the scenes.
Factories that make nylon and high-performance polymers want something that gives materials strength, flexibility, and resistance to wear. Hexadecanedioic acid finds its way into these production lines because it makes polymers tougher and longer-lasting. In my time working with a plastics manufacturer, I saw how using reliable acids like this one makes a big difference in keeping products from cracking or breaking down. It helps keep luggage wheels rolling longer, zippers zipping, and even auto engineering parts holding together after rough use.
The usefulness of hexadecanedioic acid doesn’t stop there. Cosmetic companies use it in skin care products and lotions. Its molecular shape helps improve the texture of creams. That means the blended product doesn’t separate as easily, so the cream feels smoother on skin and helps trap moisture. Doctors and pharmacists also look at it for drug delivery, since its structure can help medicines dissolve more slowly or reach certain parts of the body more effectively. This keeps the dosing steady and can possibly cut down on side effects, which matters to anyone who has to take medication regularly.
Like many chemicals, how it’s produced and handled has real consequences. Factories often make it using renewable resources like plant oils, although some still rely on petroleum-based starting materials. The shift to greener sources isn’t just about marketing—many companies genuinely want safer supply chains, especially because buyers pay attention to sustainability. Part of the appeal comes from how it breaks down relatively easily in the environment, reducing long-term pollution risk. That’s one positive compared to some persistent plastics and microbeads that stick around for years.
If there is a sticking point, it’s that large-scale production needs rigorous quality and safety controls. Regulatory bodies carefully monitor substances that go into anything humans touch or ingest. Factories must meet strict purity and environmental standards, which means frequent investment. Anyone working in or with an industrial facility gets familiar with audits and batch testing, since even small contamination can ruin large batches of finished materials.
Moving ahead, the focus will likely rest on using renewable feedstocks. Continued research on biodegradability could push hexadecanedioic acid into new types of packaging and electronics. Supporting research means more jobs for chemists and engineers, and can cut costs for consumers in the long run. Consumer attention on sustainability keeps company decision-makers honest and pushes the market toward cleaner solutions.
Hexadecanedioic acid proves that even a mouthful of a name can have straightforward, everyday importance if you look close enough.
Most folks haven’t heard much about hexadecanedioic acid, but it turns up in more surprising places than you’d imagine. This powder is a type of dicarboxylic acid—think of it as a building block for making all sorts of polymers and coatings. Some industrial processes use it. It pops up in research about plastics and resins, and in some cases, science people look at it for possible medical and cosmetic purposes.
Long chemical names start raising eyebrows once companies consider using them in creams, pills, or food. People want to know what happens if they put it on skin, swallow it by accident, or work with it at a factory. With social media now supercharging health concerns, transparency matters a lot. The FDA, the European Chemicals Agency, and similar regulators don’t mess around with this stuff. They demand real data, not just “should be safe.”
The raw truth: there’s not a massive pile of studies on hexadecanedioic acid and human safety. The available toxicology reports mostly focus on industrial-level exposure. In animal tests, high levels sometimes cause mild irritation. No trusted studies point to it causing cancer, genetic mutations, or serious allergic reactions. The Cosmetic Ingredient Review panel did check out the acid, giving it the green light in low concentrations for topical uses. That said, anyone with sensitive skin could still react.
A lot of dicarboxylic acids break down in the body in predictable ways. Based on current data, the body can process small amounts safely. Acute toxicity appears low. Chronic exposure studies are thin on the ground, so nobody should claim zero risk from regular, high-dose use.
I once toured a factory where chemical dust filled the air, and even common food flavors stung the eyes. Protective gear isn’t just for show—it really matters. Hexadecanedioic acid, like most fine powders, can irritate the nose and throat if inhaled. Good ventilation cuts back on the risks. Gloves and goggles are a given for anyone mixing or pouring chemicals.
For medical or food use, the hurdles stand even higher. Ingredients need approval for those paths, meaning extensive testing for safety and side effects, with strict purity standards. So far, no major regulatory agency has listed hexadecanedioic acid as broadly permissible in food or drugs. This should give pause to any company hoping for a shortcut.
Whenever a new ingredient pops onto the market, especially something with a name like this one, independent third-party testing makes a difference. People deserve transparency about where chemicals come from, what’s in the bottle, and any long-term risk. Digital batch tracing and QR codes on packaging could connect buyers straight to actual toxicity studies and results.
I’ve seen the positive shift in industries when they open up their test data for public scrutiny. Keeping facts out in the open builds trust. Anyone researching this acid for skincare, food, or drug use should push for more comprehensive studies, covering chronic exposure and sensitive groups. Regulators need to catch up with technology, not drag their feet.
The best way to handle any “scary-sounding” ingredient is plain language and real-world examples, not alarmist headlines. Honest answers about what we know and what still needs research build credibility. In my opinion, clear communication about chemicals and their risks plus firm oversight will always beat panic or blind trust.
Most folks probably don’t give a second thought to a molecule called hexadecanedioic acid. Yet in laboratories, manufacturing, and even medicine, this compound has a real story to tell. If you’ve ever handled substances in a chemistry class or worked with industrial materials, complex-sounding carboxylic acids like this come up enough times to remember their quirks. The chemical structure boils down to a 16-carbon chain with carboxylic acid groups capping each end—solidly in the family of dicarboxylic acids. Its formula is C16H30O4. Each end has that -COOH group, so you get symmetrical reactivity that materials chemists appreciate.
Imagine a straight alkane backbone—sixteen carbons, nose to tail. At carbon number 1, you tack on a -COOH group. At carbon number 16, another -COOH group. Between these endpoints, each internal carbon, except the terminals, grabs two hydrogens. Altogether, hexadecanedioic acid lands a spot among the “long-chain dicarboxylic acids,” stretching the molecule out and giving it unique properties. Lab texts draw it linearly: HOOC-(CH2)14-COOH. The length and those acid groups at both ends lend themselves to specific chemistry, including making polymers or serving as a metabolic substrate in rare medical pathways.
Factories and pharmaceutical labs look at hexadecanedioic acid’s structure for its range of uses. Engineers value molecules like this in polyester production or as monomers for specialty nylons. The long chain gives flexibility, strength, and resilience to the finished product. I once toured a plant making high-performance fibers for car airbags and, sure enough, their process involved fatty diacids like this one. Properties come straight from the structure—length gives flexibility, terminal carboxylic acids allow bonding into larger frameworks.
Medically, this molecule turns up for research on rare metabolic disorders. Peroxisomal disorders like X-linked adrenoleukodystrophy cause problems breaking down long-chain fatty acids, including hexadecanedioic acid. Bloodwork sometimes reveals high levels, acting as a biomarker for these conditions. Structure determines detection—most common blood tests rely on that 16-carbon backbone with its unmistakable acid caps.
Handling long-chain dicarboxylic acids always raises a few headaches—solubility remains a hurdle. You can’t just dissolve hexadecanedioic acid in water and call it a day. Tools like organic solvents or mechanical agitation make solutions workable, but every extra step means extra cost. Some companies are developing better purification and processing tools, which could mean more applications in greener plastics or medical diagnostics. A few teams in universities target biosynthesis through engineered microbes, hoping to skip over energy-heavy chemical synthesis.
Knowledge around this molecule’s chemical structure matters because it touches daily work in so many technical fields. Whether building resilient new materials or tracing rare metabolic syndromes, hexadecanedioic acid’s symmetrical design makes it an object of focus and, for now, a challenge to craft cleanly and sustainably.
Hexadecanedioic acid might sound like a mouthful, but for folks in laboratories or industries dealing with polymer goods and specialty chemicals, it pops up often enough. This powder-like solid brings some useful properties for manufacturing, yet its safety demands real attention. If anyone has ever opened a storage cabinet in a lab filled with white crystalline powders, the lessons about careful handling stick around for good reason.
From my years working with organic compounds, storing any dicarboxylic acid, including hexadecanedioic acid, should always start with the basics: dry, cool, and well-sealed. Even if the material appears stable on paper, moisture can clump powders and spark unwanted reactions. In a room with over 60% humidity, I’ve seen unopened bottles turn into hard little rocks—all because someone skipped the desiccator cabinet. Back in college, we had to pitch a whole batch because it picked up moisture and stopped working right in our experiments.
Heat poses an equal risk. High temperatures encourage decomposition and degradation, especially over months or longer. Most chemical supply datasheets recommend under 30°C, which basically means room temperature in air-conditioned spaces. Keeping the containers out of direct sunlight or away from radiators matters. I keep heat-sensitive stocks in cupboards far from vents and windows to avoid any surprises during the summer.
Original manufacturer packaging typically works best, but repackaging happens when sharing or subdividing among colleagues. Use glass containers with airtight screw caps for any carbon-chain acid; avoid basic plastics that may leach or interact with fine powder. I opt for borosilicate glass every time. For a few years, I underestimated how much cross-contamination ruined shelf life until a mentor showed me corroded container lids.
Label everything clearly—date of receipt, source, and product name. There’s nothing worse than sorting through faded jars, wondering if the contents are still usable or safe. My habit is to stick a layer of transparent tape over the label to protect it during spills, and update the inventory list regularly. This approach helps prevent misidentification or accidental mixing, which remains a common enough lab mistake.
Proper storage requires a ventilated, dedicated area. I keep acids away from reactive compounds like strong bases or oxidizers. On a bad day, failing to segregate chemicals can run up costly damage or lab shutdowns, which I’ve seen first-hand. Even stable acids like hexadecanedioic carry some hazard if stored carelessly. Gloves, goggles, and a dust mask provide a real barrier every time a container is opened, since handling powders brings inhalation risks. Accidents from cutting corners linger longest in memory.
Storing hexadecanedioic acid isn’t complicated, but respect for the chemical and vigilance about the environment make a difference. I stay in the habit of monthly checks and quick housekeeping. These routines save headaches in inventory management and keep colleagues confident in the shared workspace. For teams handling chemicals, day-in and day-out, nothing replaces the power of good preparation and a tidy cabinet.
Anyone working with chemicals knows the conversation about purity pops up constantly. At first glance, hexadecanedioic acid looks like just another name on a lengthy inventory list. But this dicarboxylic acid, with its sixteen-carbon backbone, deserves a closer inspection if your end goal isn’t simply to tick a box but to see a real-world outcome, whether that means a polymer with fewer defects or a lab process running a little smoother.
When I first got a shipment in years back for a research project, I learned fast that not every bottle labeled “hexadecanedioic acid” held quite the same thing. Grades can differ, and those differences ripple out quickly into product reliability and safety. You find technical grade and high-purity options. The technical grade works for applications like industrial plastics, where a few minor contaminants won’t sink a batch. Higher purity, sometimes called reagent or pharmaceutical grade, matters much more where trace elements could trigger unwanted reactions or complicate downstream processes.
It feels easy to grab what’s cheapest or what’s sitting on the local supplier’s shelf. In reality, I’ve watched teams lose weeks because trace impurities in a low-grade batch skewed results or forced repeated troubleshooting. For pharmaceuticals or cosmetics, contamination creeps in as a risk for regulatory rejection or, worse, patient harm. This awareness has pushed many labs and factories to demand certificates of analysis and hold suppliers to strict standards.
The chemical industry moves according to a basic logic here. Manufacturers selling to researchers, drug-makers, or those in high-precision fields invest in extra purification steps and more thorough inspections. That costs more, but it puts minds at ease. On the other hand, for uses like adhesives or lower-grade polymers, buyers often stick with technical grade, where a few extra molecules of something else cause no real harm.
Reports from markets like Statista and Grand View Research break it down pretty clearly. Big chemical suppliers sort their stock into classes, usually ranging from technical (often above 95% pure) to purified lab and pharmaceutical grades (often 98-99% or higher). These numbers might not sound like a huge gap, but just a percent or two can change everything—the melting point, the solubility, even the smell sometimes. Sometimes you see reports of metal traces or organic residue in lower grades. Unfortunately, nobody finds out until they’ve already set up a pilot run.
If you want fewer headaches, push suppliers for batch testing and purity documentation right away. This hasn’t always been standard practice everywhere. The move towards more documented, transparent supply chains began with larger buyers demanding more accountability. For a small lab, asking for the certificate early saves time and protects data. In factories, running in-house tests on new supplies before batch mixing stays smart business.
If you ever come across doubts about a particular grade, just putting two samples side by side under a microscope or through simple chromatography offers quick clarity. Some companies set up multi-sourcing agreements, picking suppliers with proven track records rather than settling on whoever offers the sharpest price point. This keeps genuine competition alive and lets end users pick up better material over time.
Purity levels of hexadecanedioic acid make a real difference. It’s not just a chemist’s obsession—it shows up as lost product, wasted time, extra maintenance, or worse if overlooked. Backing up every order with solid documentation and evidence-based selection makes investment in higher grades more than just a precaution—it’s sound risk management for anyone relying on predictable results.
| Names | |
| Preferred IUPAC name | hexadecanedioic acid |
| Other names |
1,16-Hexadecanedicarboxylic acid
C16 Dicarboxylic acid Hexadecandioic acid palmidic acid Sweetening acid |
| Pronunciation | /ˌhɛk.səˌdiːkə.nɪˈdiː.ɒɪk ˈæs.ɪd/ |
| Identifiers | |
| CAS Number | 505-57-7 |
| Beilstein Reference | 1209979 |
| ChEBI | CHEBI:18069 |
| ChEMBL | CHEMBL1407 |
| ChemSpider | 21476442 |
| DrugBank | DB03912 |
| ECHA InfoCard | CHEMICAL INFOCARD: 100.017.338 |
| EC Number | 204-664-4 |
| Gmelin Reference | 162135 |
| KEGG | C08285 |
| MeSH | D006530 |
| PubChem CID | 10464 |
| RTECS number | MO2450000 |
| UNII | Q39G971Y2S |
| UN number | UN2811 |
| Properties | |
| Chemical formula | C16H30O4 |
| Molar mass | 286.44 g/mol |
| Appearance | White powder |
| Odor | Odorless |
| Density | 0.97 g/cm³ |
| Solubility in water | Insoluble in water |
| log P | 3.99 |
| Vapor pressure | 1.52E-9 mmHg at 25°C |
| Acidity (pKa) | 4.89 |
| Basicity (pKb) | 1.95 |
| Magnetic susceptibility (χ) | -99.7e-6 cm³/mol |
| Refractive index (nD) | 1.475 |
| Viscosity | Viscous liquid |
| Dipole moment | 3.74 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 309.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -894.8 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -8884.7 kJ/mol |
| Pharmacology | |
| ATC code | A16AX15 |
| Hazards | |
| Main hazards | May cause respiratory irritation. Causes skin irritation. Causes serious eye irritation. |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS07,GHS08 |
| Signal word | Warning |
| Hazard statements | H317: May cause an allergic skin reaction. |
| Precautionary statements | Precautionary statements: P261, P280, P305+P351+P338, P337+P313 |
| Flash point | 215 °C |
| Autoignition temperature | 330 °C (626 °F; 603 K) |
| Lethal dose or concentration | LD50 (oral, rat): > 5000 mg/kg |
| LD50 (median dose) | LD50 (median dose) of Hexadecanedioic Acid: "5000 mg/kg (rat, oral) |
| NIOSH | NA5440000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | General purpose polyamide/copolyamide |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Dodecanedioic acid
Octadecanedioic acid Terephthalic acid Sebacic acid Adipic acid |