
Ethyl salicylate first caught the attention of chemists in the late 1800s when interest in aromatic esters began to grow. Laboratories searched for ways to synthesize compounds that offered pleasant fragrances and potential medicinal value. Early use focused on fragrance manufacturing, exploiting its sweet, wintergreen-like aroma. Over decades, it carved out a niche in both perfumery and pharmaceuticals. The work of Oswald Schmiedeberg and collaborators in the late 19th century drove forwards both the understanding and practical uses of salicylate compounds, helping to distinguish between the effects of methyl and ethyl esters on biological systems. By the early 20th century, factories across Europe put ethyl salicylate to use in flavoring and topical analgesics. This long-standing role as both a fragrance molecule and a medicinal intermediate continues, as the compound remains under study for newer applications.
Ethyl salicylate stands out for its dual functionality in scent and chemistry. Anyone who has smelled the gentle sweetness of wintergreen or certain chewing gums has caught a whiff of it. In product form, it appears as a clear, colorless or slightly pale yellow liquid. Fragrance makers and pharma firms buy it in bulk to blend with other oils, where it acts as a top note in perfumery or a mild analgesic ingredient in oral health products. Manufacturers rely on its chemical stability and defined sensory profile to maintain batch consistency. Although its global tonnage lags behind heavier industrial esters, demand keeps steady, with spikes tied to new research and consumer trends.
At room temperature, ethyl salicylate flows as a lightweight liquid. It boils around 254°C, much higher than water, which makes it sturdy under typical manufacturing conditions. Its density sits close to 1.1 grams per milliliter. It dissolves well in alcohol and organic solvents and stays almost invisible in water—collecting only tiny concentrations, thanks to its limited polarity. Chemo-sensors highlight a strong aromatic structure with an ester group linked to a salicylate backbone. This structure gives it both its recognizable scent and its chemical resilience. Breaking the molecule down in a lab takes significant heat or base; under standard storage conditions, it resists light and oxygen, only slightly yellowing after years on the shelf.
Suppliers detail purity, typically offering products above 99% by GC analysis. Regulatory bodies like the European Chemicals Agency and the US FDA set guidelines for allowable impurities, especially if the product goes into food or medicine. Labels need to declare the chemical name, batch number, manufacturing date, and storage guidelines. Many manufacturers add warnings about skin and eye contact, urging operators to use PPE at production sites. Clear GHS-compliant pictograms show up on every drum—a response to modern workplace safety expectations. For pharmaceutical or food-grade supplies, certificates of analysis accompany each shipment, verifying not just purity but the absence of heavy metals and potential allergens.
The most common approach to synthesize ethyl salicylate uses the classic Fischer esterification reaction. Salicylic acid and ethanol react under acidic conditions, usually with concentrated sulfuric acid as a catalyst. The process involves heating the mixture to remove water, which forces the equilibrium toward ester formation. Factories have scaled up this reaction by using distillation to pull out ethanol and water as they form, making higher yields possible. After the reaction, the crude product gets washed, neutralized, and dried with salt before final distillation. Smaller specialty batches sometimes rely on newer enzymes or solid acid catalysts to improve environmental profiles and cut waste acid production.
Ethyl salicylate reacts like a typical aromatic ester. Under strong hydrolysis with base, it breaks into salicylic acid and ethanol, a trick often used to reclaim starting materials or confirm identity during quality tests. Nitration or sulfonation modifies the aromatic ring, producing intermediates for dyes or more potent medicinal agents. Chemists have adjusted the ester group, swapping ethyl for longer chains or bulkier alcohols to develop esters with altered scents and improved skin absorption. Every so often, research labs attempt hydrogenation or halogenation, searching for novel analogs with unexpected properties. Despite the fun of experimentation, the core structure doesn’t change easily, giving the molecule its stability and ongoing usefulness.
Within reference texts and chemical catalogs, ethyl salicylate pops up under several names. Commonly, it appears as “Salicylic acid ethyl ester” or “2-hydroxybenzoic acid ethyl ester.” Some older texts still call it “Oil of Wintergreen (synthetic),” though current terminology makes a sharp divide from the methyl variant. Global suppliers might label it by the CAS number 118-61-6, smoothing out translations. In pharma and food circles, the EINECS number or E code might appear, though its use in foods has dropped in some regions due to shifts in safety modeling and flavor preferences.
Manufacturing and handling require solid safety habits. Ethyl salicylate can irritate eyes and skin, and concentrated vapors affect factory workers over time. Safety data sheets place strong emphasis on proper ventilation and wearing gloves. Food and pharma makers keep residue well below caps set by EFSA and FDA, recognizing that chronic exposure in sensitive populations, especially children, could result in side effects similar to salicylate drugs. Fire risks arise only during bulk storage because the liquid carries a flashpoint over 100°C, so routine conditions seldom light it up. Training programs follow strict protocols for spill containment and waste treatment, underscoring personal and environmental protection.
Fragrance and food industries have made the most noise about ethyl salicylate over decades. It earns top billing in synthetic wintergreen flavors, perfumes, and certain toothpaste. Its low toxicity profile appeals to flavorists working on topical products, mouthwashes, and temporary pain relief gels. Pharmaceutical companies explore its role as a masking agent for bitter drug flavors. Chemists have found uses in laboratories as a model compound for studying ester reactions or drug metabolism. More recently, there’s interest in biomed—using it as a pro-drug to deliver salicylate ions slowly through skin or mucous membranes, hinting at uses in advanced drug delivery systems. Boutiques in natural products and essential oils sometimes lean on it for safe aromatic blends.
Modern research digs into new formulations and delivery systems. Teams work on microencapsulation to improve stability in hot climates or extend shelf life in challenging environments. Biomedical studies push to uncover stronger anti-inflammatory actions or more controlled release, which could move ethyl salicylate deeper into pain management. Analytical chemists test modified derivatives for more appealing scent profiles that avoid regulatory landmines tied to certain allergens. In green chemistry, researchers look for biosynthetic routes using engineered microbes or enzyme catalysis, cutting reliance on harsh acids and boosting production efficiency. Collaborative projects across Europe and Asia tackle toxicological uncertainties with long-term in vivo studies, responding to rising global scrutiny around chemical exposures in daily life.
Toxicologists have studied ethyl salicylate for decades, especially after the aspirin-related Reye’s syndrome scare upped scrutiny on all salicylate derivatives. Acute toxicity in healthy adults stays low, with only large doses causing notable problems—symptoms align with those seen in salicylic acid overdoses: nausea, dizziness, ringing in the ears, and, in extreme cases, metabolic acidosis. Certain populations, especially young children and those with metabolic disorders, show higher sensitivity, leading to regulatory limits in food and topical products marketed for kids. Evaluations from EU and US agencies regularly reaffirm the need for caution, even as typical consumer exposures remain well below worrisome levels in the current market.
The next phase for ethyl salicylate draws from the surging interest in “safer scent” formulations and more natural-feeling drug delivery systems. With environmental regulators tightening on waste effluents and consumer pressure up for “clean label” ingredients, manufacturers will invest heavily in greener synthesis and safer alternatives. Scalable biocatalysis could come to dominate production, slashing the carbon footprint and dodging acid waste. In pharma, much of the focus shifts toward creating dual-acting agents that harness the anti-inflammatory edge of salicylates without the side effects linked to older compounds. Personal care producers and flavor houses hunt for tweaks that boost stability and tailor fragrance complexity for niche markets. Across all fields, ongoing research keeps a close eye on chronic toxicity and allergenic potential, balancing innovation with a continued respect for safety.
Step into most pharmacies or stroll through a perfume aisle, and ethyl salicylate has probably played a role in what you smell. This compound pops up in all sorts of products because of its sweet, wintergreen-like scent. Perfumers love it for the rich, minty background it creates in colognes and lotions. The same property that makes you pause and enjoy that crisp, fresh aroma in personal care items can be traced back to ethyl salicylate.
Beyond fragrance, chewing gum and certain candies also benefit from ethyl salicylate because it gives them a distinct, cooling mint flavor. Drinks and foods sometimes carry traces of it, mostly for flavor enhancement. The FDA places ethyl salicylate on its list of substances generally recognized as safe at low levels, but it’s always a good idea to stay informed about what you’re eating and what goes into popular treats.
Pharmacists and manufacturers have relied on ethyl salicylate due to its close relationship with aspirin. Both molecules stem from salicylic acid, a compound that has helped relieve pain and inflammation for more than a century. Ethyl salicylate sometimes finds its way into liniments, balms, and ointments targeting muscle soreness. These products use the compound for its mild pain-relieving qualities and its role as a counterirritant—the familiar “soothing heat” feeling from some rubs and patches actually comes from this ingredient.
Safety isn’t just a suggestion in health products; it’s an expectation. Evidence points to low toxicity with responsible use, but people who face allergies to aspirin or its relatives need to watch ingredient lists for ethyl salicylate. Even topical use can pose a problem for those with sensitivity, so transparency in labeling helps customers avoid adverse reactions. Overuse or misuse, especially in large doses, may bring risks. Back in school, I remember learning about salicylate poisoning—rare but not impossible if someone ingests too much through medication or by accident. The lesson stuck with me: moderation and knowledge really do matter.
In industrial circles, ethyl salicylate’s role isn’t limited to seducing noses. Scientists use it when testing new solvents because of its clear chemical makeup and predictable qualities. It brings a consistency needed for reliable experiments, and the food industry values it as a tested flavor agent. It’s simpler to control than straight-up methyl salicylate (oil of wintergreen), given differences in potency and interaction with the body.
One thing stands out: industries continuously need to balance flavor and aroma with consumer trust. Relying on tested, established ingredients, and clearly communicating their presence, builds credibility. Regular safety reviews, smart packaging, and honest ingredient lists help prevent misuse and foster informed choices for everyone, from flavor chemists to parents choosing lotion for their kids.
Ethyl salicylate’s journey from plant origins to global production shows the intersection of science, tradition, and daily life. As product formulas get more complex and consumers ask sharper questions, openness about chemical ingredients enjoys renewed importance. People appreciate knowing why a familiar aroma or flavor shows up in their living rooms or lunchboxes. Making science approachable in plain language takes the mystery out and keeps trust strong—something everyone deserves.
Ethyl salicylate carries a sweet, wintergreen scent. Manufacturers use it to add fragrance to perfumes, flavor to oral care products, and sometimes a touch of scent to topical creams. The name sounds intimidating, though the molecule itself has a long record in personal care formulas.
Dermatologists and chemists recognize ethyl salicylate as a derivative of salicylic acid. On healthy skin with no open wounds or chronic sensitivity, the scientific community considers it low-risk at the small concentrations usually found in cosmetics. Research in the International Journal of Toxicology documents minimal skin irritation from ethyl salicylate at levels up to 5%. The Cosmetic Ingredient Review (CIR), a U.S.-based scientific panel, reviewed available safety data and deemed it safe in rinse-off and leave-on formulations under typical use conditions.
Still, safety doesn’t mean zero concern for everyone. Some individuals become sensitized to salicylates after repeated use, especially if their skin already flares up easily. Salicylate allergies show up as rashes, itching, or hives. People with diagnosed aspirin allergies or uncontrolled eczema should avoid formulas containing salicylate derivatives, including ethyl salicylate. Chemical burns are rare, though not unheard of, if someone with very delicate skin applies it in high doses.
As a former pharmacy technician, I've watched friends and customers deal with mysterious red, itchy patches after using new creams or lotions. Many people skip the patch-testing step, even though a dab on the inner arm saves a lot of hassle later. If redness, swelling, or stinging shows up after a few hours, washing the area and switching to a different product makes the day much less stressful. Simple, practical steps—check the ingredient list, use a sample size, and monitor reactions—separate a pleasant skincare experience from a rashy disaster.
Groups like the U.S. Food and Drug Administration and the European Commission keep a close eye on cosmetic safety. Ethyl salicylate earns approval in leave-on and wash-off products as long as brands stick to the guidelines. Most creams and lotions contain only trace amounts, well under levels that cause skin reactions in healthy adults. Reputable brands disclose this ingredient clearly on packaging and websites.
Concerns sometimes pop up about absorption. A healthy adult’s skin acts as a shield, so large-scale absorption remains unlikely at the doses found in skin care. No one should apply these products to broken skin, though—barrier damage means greater risk for irritation. Experts also advise pregnant and breastfeeding individuals to talk with doctors before using any product containing salicylates, to rule out risk to developing babies.
People chasing healthy skin often get lost in labels and jargon. Reading up on ingredients, listening to the body’s signals, and reaching out to a dermatologist for persistent issues pay off. Allergy-prone individuals can stick to fragrance-free, hypoallergenic options. Those with cautious skin won’t go wrong using plain moisturizing ingredients rather than chasing every new scented formula. Everyone should keep in mind that mixing too many actives at once—like salicylates with retinoids or alpha hydroxy acids—increases the odds of skin irritation.
Safer skin comes from careful choices and plain old listening to what feels right. Ethyl salicylate has earned its place in trusted beauty and personal care products, but respect for personal differences outweighs trends and marketing. Anyone with questions about a new ingredient deserves a straightforward answer, not just a fancy name on a label.
Ethyl salicylate often pops up in daily life. The chemical gives certain perfumes a wintergreen smell and sometimes shows up in flavorings or used in topical medicines. Having worked with pharmacy shelves and ingredient lists in skin products, I always check for salicylates if someone mentions allergies or odd skin reactions.
Salicylate sensitivity runs in my family, so I’ve seen more than a few upset stomachs, hives, or rashes from aspirin or mint-flavored drinks. Ethyl salicylate can act in similar ways. People with asthma or a history of aspirin reactions can run into trouble after using products with this ingredient. Common symptoms include itching, redness, swelling, or a sudden runny nose and wheezing. For some, a single encounter brings on a full-body reaction. I had a friend who developed blisters using a fragrance oil that listed “salicylate”—after checking, ethyl salicylate was the source.
Skin irritation comes up often when looking at the safety of this compound. Anyone who ends up with a product spilled on their hands might notice redness, peeling, or a burning feeling. In labs, technicians need gloves because repeated or concentrated exposure creates sores. Eyes don’t fare much better, and getting ethyl salicylate in the eyes causes tears, pain, and even blurred vision until the chemical is washed out thoroughly.
Not all the risk stays on the outside. Ethyl salicylate, like other salicylates, can be absorbed through the skin or mucous membranes. Accidentally swallowing or inhaling enough can upset the body by mimicking high-dose aspirin exposure. Some people report nausea, vomiting, ringing in the ears, and feeling confused. There’s even a name for this group of symptoms: salicylate poisoning. Doctors see it most often in kids who eat flavored toothpaste or in adults handling strong concentrations at work. Too much salicylate stresses the kidneys and can lead to dangerous acid build-up if not caught early.
People often underestimate how many products contain these compounds. To avoid surprises, check for ethyl salicylate on labels, especially if allergic to minty flavors, aspirin, or have chronic respiratory problems. At work, using gloves and eye protection keeps most exposures at bay. I learned quickly to use air flow systems in the shop after headaches and watery eyes got too common.
For parents, locking up anything minty in smell or taste is smart, and teaching kids not to sample their toothpaste makes sense too. Doctors recommend stopping use and seeking help for fast swelling or trouble breathing. For milder symptoms, rinsing with lots of water usually relieves irritation on skin or in eyes.
Newer research keeps an eye on allergies and toxicity of salicylates, especially as flavors and fragrances keep expanding into more household items. Better labeling and public awareness around these risks go a long way, especially for anyone living with allergies or chronic health issues. Like many substances used in everyday products, knowledge helps avoid hidden trouble, and makes the necessary choices to steer clear of reactions.
Ethyl salicylate carries a sweet, wintergreen aroma, popping up everywhere from perfumes and chewing gums to pharmaceuticals. Behind that pleasant scent lies a chemical story that calls for proper care. Ethyl salicylate, like many organic esters, responds to heat, air, and moisture in surprisingly strong ways. As someone who's spent time in both labs and storerooms, overlooking these details has landed me with degraded samples and wasted resources more often than I care to admit.
Direct sunlight can quickly ruin ethyl salicylate’s aromatic qualities. I’ve seen clear bottles go cloudy and start to give off odd smells after just a few afternoons by a sunlit window. Keeping this compound in an amber glass container makes a difference. These bottles block out most UV rays and keep the contents stable for much longer. Plastic doesn’t cut it—light and certain plastics don’t mix well, and cross-reactions can creep in, tainting both the chemical and container.
Temperature swings transform what should be an easy storage job into a headache. In one research facility, a small batch left near a heat vent broke down much faster compared to those stored at steady room temperature. Ethyl salicylate holds up well in cool, dry places, far from radiators or tropical heat. Temperature above 25°C could trigger unwanted hydrolysis or break down the ester bond, producing salicylic acid. That change not only ruins the desired scent but may also introduce irritants.
Even pros can forget about moisture, which creeps in through loose caps or careless handling. Humidity doesn’t just dull the flavor or aroma—it breaks the compound down, fast. A desiccator, or a shelf lined with silica gel packs, helps keep the environment dry. Gaps in the sealing system, whether it’s a cracked cap or a worn stopper, expose the chemical to air as well. Oxygen turns into an invisible enemy, slowly oxidizing ethyl salicylate and wrecking its desired properties. During one particularly humid summer, I watched an entire shelf’s worth of chemicals succumb to poor seals and moisture, turning product after product into useless sludge.
Mislabeling materials and poor organization set up more accident traps than any single technical flaw. Once, a mislabeled bottle led to confusion and misplaced blame, all because simple identification protocols weren’t followed. Every bottle deserves a durable label, marking the contents, concentration, and date of receipt. Clear labeling not only prevents mix-ups, but makes audits and inventory smooth work.
Training isn’t just for new arrivals in the lab or warehouse. Regular reminders about proper storage procedures, chemical incompatibilities, and the value of prompt inventory checks stop preventable losses before they start. During routine safety checks, team members often discover expired or compromised chemicals, which can be safely disposed of before causing more trouble.
Safe storage of ethyl salicylate protects against unnecessary risks and helps ensure product quality. Basic steps—cool, dry environments, protection from light, airtight seals, good labeling, and solid training—work together to cut down on wasted materials, time, and the chance of dangerous reactions. In both commercial and research spaces, getting these habits right covers far more than compliance; it reflects care for the work, the product, and everyone nearby.
People spot “salicylate” on a label and picture the same thing, but in the world of chemistry, small shifts make a big difference. Take ethyl salicylate and methyl salicylate. Both trace their roots to salicylic acid, the molecule aspirin comes from. That’s where the similarities end. Once you swap that “methyl” for an “ethyl,” you’re dealing with new smells, flavors, and uses. Treating these two as one and the same leads to mix-ups that can prove costly, if not downright dangerous.
Methyl salicylate often comes to mind first. Most folks know it as wintergreen oil. It delivers that signature punch found in gum, toothpaste, and muscle rubs. It’s easy to find on the shelves mixed into creams that warm sore shoulders or flavoring your childhood pink bubble gum. Methyl salicylate packs a very particular smell and a high level of absorption through the skin. This makes it useful for topical pain relief—but also a risk for toxicity, especially in children.
Now, ethyl salicylate changes things up. Swapping the methyl for an ethyl shifts the scent to something more like sweet, almost grape-leaning notes—think of artificial grape popsicles or perfumes with a soft, powdery finish. It isn’t a go-to in pain rubs. Instead, it pops up in food as a flavoring, perfumes, and specialty products. Ethyl salicylate doesn't rush through the skin in the same way. This makes accidental poisoning less likely, though that doesn’t mean the green light for careless use.
Anyone who’s grabbed the wrong bottle from a lab shelf knows the nerves that hit as soon as you realize something’s off. In practice, using methyl salicylate in place of ethyl salicylate—or the other way around—can cause real headaches. A baker hoping for a gentle grape note ends up with the medicinal blast of wintergreen. A pharmacist expecting mild flavor lands strong skin irritation. More concerning, some still believe natural equals safe. A teaspoon of methyl salicylate can prove deadly to children, according to poison control reports.
These aren’t just chemical footnotes; they’re safety issues. Both chemicals serve unique roles. The choice hinges on a real understanding of what each version brings to the table. Greater awareness stops mix-ups before they start.
Folks working in food and drug manufacturing need solid training. Workers in these industries handle hundreds of ingredients, many with similar-sounding names. Big, clear labeling and regular safety briefings can slash human error. Retailers also play a role. Clear ingredient lists on products help families make better decisions about what ends up in their homes or on their skin.
Consumers shape the conversation, too. Taking the time to check labels, learn about common additives, and ask questions gives power back to the shopper. Trust builds through transparency matched with real education. There’s no room for confusion when health and trust are at stake. Small details—the choice of ethyl or methyl—matter more than most think.
Having worked with both these compounds in college lab sessions, one mistake stood out: underestimating what the right label means. It took one strong whiff and a minor headache to never make that mistake again. These experiences stick, and they remind us that attention to detail beats assumptions every time.
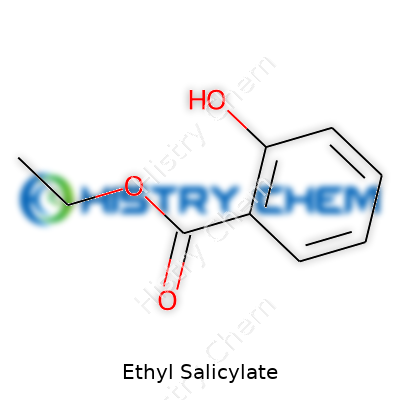
| Names | |
| Preferred IUPAC name | ethyl 2-hydroxybenzoate |
| Other names |
Ethyl 2-hydroxybenzoate
Salicylic acid ethyl ester |
| Pronunciation | /ˈiːθɪl səˈlɪsɪleɪt/ |
| Identifiers | |
| CAS Number | 118-61-6 |
| 3D model (JSmol) | `Ethyl Salicylate JSmol 3D model string`: ``` ethylsalicylate ``` You can use this string in JSmol-based molecular viewers to load the 3D model of Ethyl Salicylate. |
| Beilstein Reference | 1369789 |
| ChEBI | CHEBI:4915 |
| ChEMBL | CHEMBL141060 |
| ChemSpider | 19720 |
| DrugBank | DB14182 |
| ECHA InfoCard | 100.007.799 |
| EC Number | 202-259-7 |
| Gmelin Reference | Gmelin Reference: 120789 |
| KEGG | C10452 |
| MeSH | D013675 |
| PubChem CID | 6938 |
| RTECS number | VO4725000 |
| UNII | 3B2U3XRS0Y |
| UN number | UN1179 |
| Properties | |
| Chemical formula | C9H10O3 |
| Molar mass | 200.22 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | fruity; wintergreen |
| Density | 1.14 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | 2.49 |
| Vapor pressure | 0.03 mmHg (25°C) |
| Acidity (pKa) | 12.7 |
| Basicity (pKb) | 13.7 |
| Magnetic susceptibility (χ) | -50.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.538 - 1.541 |
| Viscosity | 2.13 cP (20°C) |
| Dipole moment | 2.69 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 235.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -439.1 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3387 kJ/mol |
| Pharmacology | |
| ATC code | N02BA05 |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02,GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319 |
| Precautionary statements | P210, P261, P264, P271, P273, P280, P301+P312, P302+P352, P304+P340, P305+P351+P338, P312, P332+P313, P337+P313, P403+P235, P501 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | Flash point: 110°C |
| Autoignition temperature | 555°C |
| Explosive limits | Explosive limits: 1.1–6.8% |
| Lethal dose or concentration | LD50 (oral, rat): 1980 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 920 mg/kg |
| NIOSH | SA 9260000 |
| PEL (Permissible) | PEL (Permissible exposure limit) for Ethyl Salicylate: 5 mg/m³ (ceiling, skin) |
| REL (Recommended) | 0.10% |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Salicylic acid
Methyl salicylate Aspirin Sodium salicylate Phenyl salicylate |