
Delta-Valerolactone grew out of a wave of curiosity-driven research into lactones that swept through organic chemistry in the early twentieth century. Chemists looking to understand the building blocks of nature soon realized that these cyclic esters—especially those like delta-valerolactone—could unlock new tools in both synthesis and industry. Early experiments often used caproic acid with careful distillation, yielding a versatile monomer with broad potential but little initial fanfare. Over the decades that followed, improvements in analytical techniques, demand for biodegradable plastics, and creative organic syntheses nudged delta-valerolactone out of the margins and into a broader industrial spotlight.
Delta-Valerolactone, known for its five-membered ring lactone structure, goes to work in polymer industries, specialty chemicals, and labs worldwide. Researchers and manufacturers value its balance between reactivity and stability, giving it a role in producing biodegradable plastic, adhesives, flavors, and even pharmaceuticals. Its unique ring structure makes it a reliable building block for polyesters, which fit cleanly into today’s push for greener chemistry. Chemists prize chemicals that stay manageable on the bench or in a tank, and delta-valerolactone has developed a reputation for being just that kind of compound.
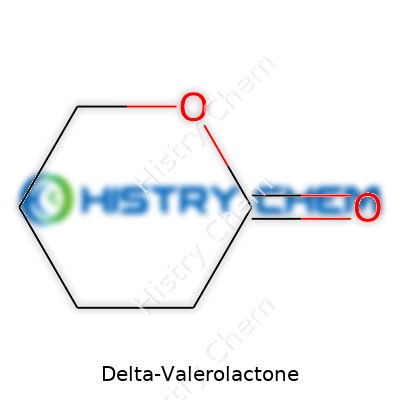
Delta-Valerolactone presents as a clear, colorless liquid at room temperature, with a faint, pleasant odor sometimes likened to coconut or fresh dough. Its molecular formula—C5H8O2—tells only part of the story. The lactone ring brings moderate hydrophobicity and a boiling point of about 207°C, but its solubility in most organic solvents offers flexibility for chemists. It has a refractive index near 1.45, density around 1.04 g/cm³, and a melting point hovering close to -31°C. One of its most intriguing aspects is how its ring can be easily opened under the right conditions, making it invaluable for further chemical transformations.
Reliable labeling should clearly state purity (often 98% or higher for lab work), water content, storage temperature, CAS number (108-29-2), and the method of stabilization if any is used. Quality standards tracked by global supply chains require strict documentation. A label that falls short risks batch failures in both industrial and research settings. Shelf life matters—oxidation or unintentional polymerization can degrade product utility fast. Packaging tends to favor amber bottles or steel drums with lined interiors, all bearing regulatory hazard statements and recommended handling procedures in plain terms to avoid accidents.
Early methods started with 4-hydroxyvaleric acid, cyclized under acidic conditions. Modern industrial syntheses scale that up through Baeyer–Villiger oxidation, starting from valeric acid precursors. Catalysts optimize yield and control unwanted by-products, but everything comes down to balancing cost and environmental burden. Solvent recycling, process intensification, and waste minimization now steer most plant operators. Demand for greener processes has spurred new routes, including biotechnological fermentative methods that convert renewable feedstocks into the lactone. These production tweaks can help shrink the environmental footprint that chemical manufacturing often leaves behind.
Delta-Valerolactone distinguishes itself with that reactive lactone ring. Break open the ring with a base or an alcohol, and you get access to a range of polyesters or hydroxyacids. Initiators like tin(II) octoate and catalysts like methanol bring out different properties in the resulting polymers, allowing manufacturers to target everything from slow-release drug carriers to tough, flexible plastics. Chemists chasing new materials often revisit the same core reaction: ring-opening polymerization. Substitution on the ring or chain extension—whether for a new plastic or a pharmaceutical intermediate—pushes into new territories regularly. It’s the dependable workhorse at the core of many molecular innovations.
Delta-Valerolactone hides behind a handful of names: δ-Valerolactone, 2-oxonanone, 5-oxyhexanoic acid lactone, and sometimes even “dihydro-2(3H)-furanone.” Across different countries or company catalogs, product codes or commercial names can crop up, but the backbone remains the same. For procurement or regulatory paperwork, matching the right synonym to the right Safety Data Sheet avoids mix-ups and keeps operations moving smoothly.
Handling delta-valerolactone demands close attention to ventilation and personal protective equipment. Fumes irritate skin and eyes, and ingestion or inhalation can set off a range of acute symptoms. Emergency protocols emphasize eye washes, gloves, and splash guards above everything else. Industry safety data aligns with conventions from OSHA, REACH, and GHS, meaning that labeling must reflect both acute and chronic hazard potential. Proper storage—away from heat, flame, and strong bases—limits accidental polymerization or dangerous decomposition. Training staff on safe transfer, spill response, and waste disposal every few months doesn’t just check a regulatory box—it protects the health of everyone on the shop floor.
Delta-Valerolactone’s core advantages show best in the growing world of sustainable plastics. Poly(valerolactone) and related polyesters degrade in soil or compost, offering a welcome alternative to petroleum-derived plastics still piling up in landfills. Medical device engineers shape its polymers into biodegradable sutures and drug delivery systems; adhesive makers reach for it to adjust tackiness, and paint formulating groups like the way it can modify viscosity without adding VOCs. The world’s evolving regulatory landscape, with a sharper eye on microplastic pollution, lays out a path for lactone-based chemistry that traditional plastics may never follow. People don’t see the monomer, but they encounter its results everywhere from hospital clinics to fast-food take-out.
Current research pivots around new catalysts that accelerate polymerization, improve control, or enable functional group “click” reactions on the polymer backbone. Biotechnologists work on metabolic engineering in yeast or bacteria to supply renewable delta-valerolactone, tracking carbon flows and yields to bring down cost. Teams working on medical devices and drug formulations test copolymers and block polymers in pursuit of slower or more predictable biodegradation. Computational chemists run simulations trying to predict chain length distribution and mechanical properties, feeding their findings back to bench chemists and plant operators alike. Academic and private sector teams keep up a steady drumbeat of patent filings on everything from packaging to electronics.
Animal studies and in vitro tests show that delta-valerolactone breaks down into relatively safe byproducts, but repeated skin exposure or inhalation can still harm workers. Research groups examine long-term exposure for possible irritancy and sensitization, aiming for clear regulatory guidelines and threshold limit values that protect health. Data gaps still exist on chronic risk, especially for populations with underlying conditions or high sensitivity to solvents, so new toxicity studies continue to earn funding and regulatory attention. Waste treatment teams look at post-consumer breakdown, mixing delta-valerolactone-based polymers with soil microbes or municipal compost and checking for build-up of any persistent or toxic intermediates.
Future demand for delta-valerolactone will come from the intersecting needs for greener polymers, biodegradable packaging, and new medical technologies. Chemists focus on making synthesis more sustainable, with less reliance on hazardous solvents or mineral acids. Biorefineries and plant-based feedstocks line up with legislative support for renewable chemicals, shifting the economics in favor of lactone-based monomers. Research partnerships grow as companies aim to leapfrog standard plastics with smarter polymers that sense, respond, or selectively degrade. Regulatory agencies and consumers alike keep asking for transparency—clearer labeling, cleaner processes, and reliable endpoints—pushing producers to improve not just what they make, but how they make it. With the right attention to safety, research, and responsible scaling, delta-valerolactone has the potential to drive meaningful progress in more sustainable material science and industrial chemistry.
Delta-Valerolactone stands far from the public spotlight, yet it gets shaped into plenty of household and industrial goods. This simple, five-membered lactone acts as a building block in chemistry labs and on factory floors. You’ll seldom see its name on product labels, but chemists appreciate its versatility.
The real value comes out in the way it forms into polyesters and copolymers—plastics that give structure to packaging, medical implants, and coatings. For example, when combined with caprolactone or glycolide, delta-valerolactone becomes part of the backbone in biodegradable plastics. This matters, because durable packaging generally lingers in landfills, yet mixing in delta-valerolactone shifts the equation toward safe breakdown in the environment.
Delta-valerolactone’s simple ring makes it easy for scientists to open up and string together. The process, called ring-opening polymerization, lets manufacturers tweak the final properties. Mixing in a bit of valerolactone changes flexibility, heat resistance, and the rate at which a product will break down. The medical field leans on this flexibility. Absorbable sutures and controlled-release drug devices rely on polyesters built partly from valerolactone, letting the body absorb the material safely after serving its job.
Years ago, researchers would run shelf tests on new biodegradable plastics, and adding delta-valerolactone made their prototypes more workable. It lets the team avoid brittle plastics and opens up new ways for chemists to connect molecules. In my own work with polymer samples, swapping different lactones taught me how even small shifts change the whole batch.
As more companies look for ways to cut plastic waste, demand for smart “bioplastics” keeps growing. Delta-valerolactone plays a part often missed by folks outside the lab. Manufacturers studying compostable shopping bags and food packaging keep reaching for it, since it helps meet strict biodegradation standards.
Big companies once stuck with petroleum-based ingredients, but the industry shift toward renewable resources opened up new paths. Delta-valerolactone can be made from biomass, not just fossil fuels. This switch reduces carbon footprints. Switching the raw source puts pressure on supply chains to trace materials and support greener chemistry. Researchers and businesses need cooperation from governments and global organizations to keep these sustainable supply chains honest and affordable.
The hurdles aren’t all chemical—regulatory paths get tangled, patents sit on crucial chemical modifications, and labs need funding to scale up production based on greener technology. Scientists have shown progress in using bacteria to make delta-valerolactone, which could cut out harsh chemicals in synthesis altogether. To get from high-cost specialty chemical to mass market staple, companies ought to invest in both process innovation and public funding support for pilot plants.
Delta-valerolactone may not show up in headlines often, but as more people demand safe and sustainable materials, the hidden chemistry starts to matter. Years spent handling materials in the lab remind me—behind every ordinary object, a string of decisions built on the modest work of compounds like delta-valerolactone makes the complicated world around us possible.
Beneath the unfamiliar name, delta-valerolactone carries the formula C5H8O2. This simple arrangement guides its entire role in lab settings and, down the line, in manufacturing and beyond. Looking back to university days, anyone who's tried to synthesize or work with lactones remembers there’s never just “one” type. Delta-valerolactone stands out because of its five-membered ring—shaped by those five carbons, eight hydrogens, and two oxygens locking together in a structure that balances flexibility and reactivity.
Understanding a chemical formula like C5H8O2 isn’t just for chemists. It means something across research, production, and environmental circles. In a world where plastic pollution fills headlines, delta-valerolactone steps up as a possible building block for biodegradable plastics. One of my earliest encounters with its practical side came during a project on green polymers. Because of its ring structure, delta-valerolactone can open up and join in chains, acting as a monomer for new, eco-friendlier plastics. That single fact—thanks to its formula—fuels a lot of real hope in reducing long-lived plastic waste.
The numbers in its formula aren’t just data on a label. They reveal reactivity and synthesis options that shape entire industries. Its two oxygen atoms are what help form its lactone (cyclic ester) structure—making delta-valerolactone more reactive than basic hydrocarbons, but still stable enough to store and use safely. College chemistry courses drill formulas into students for a reason. Over years working in R&D, shortcuts never help. Knowing that precise formula means a chemist isn’t mixing up gamma-valerolactone or another lactone during synthesis. There’s plenty of room to make a mistake if the formula gets glossed over, and wrong ingredients bring expensive setbacks and missed deadlines.
Delta-valerolactone’s value is tied to its synthesis. Most of it comes from renewable sources, like the fermentation of plant sugars, or from petrochemicals. Each route offers pros and cons. One major obstacle: plant-based production still costs more and struggles to reach industrial scale. Several startups and academic labs are working on enzymes and fermentation tweaks to push that price down and boost yield. That kind of work matters because wide adoption only comes once it competes with cheaper, less sustainable alternatives.
Another concern: safety in production and application. Its chemical stability helps, but everyone from lab techs to manufacturers pays close attention to handling and storage guidelines. Most incidents happen not because a substance like delta-valerolactone is “dangerous” on its own, but because of shortcuts or misunderstandings about its properties. High school science teachers spend hours on material safety data sheets for this reason. Continuous education and real-world, scenario-based training stick with people better than a quick lab briefing.
Looking past basic theory, the formula for delta-valerolactone shapes real conversations about sustainability, innovation, and cost. Each atom has a purpose, laying out a path for bio-based plastics and safer industrial use. Better access to this raw material pushes industries toward lower-impact alternatives. It isn’t hype to say understanding formulas drives safer labs and greener choices—the real key is getting out of the textbook and into the practical world where those choices count.
Delta-valerolactone sounds like something you’d find in a high school chemistry set, stacked on a dusty shelf. In reality, it shows up in chemical labs and in the world of polymers. People use it to make plastics or as a stepping stone in advanced manufacturing. News about chemical safety keeps popping up for good reason. Even basic compounds sometimes cause big problems if people don’t treat them with care.
The big question usually circles back to toxicity. If you handle this stuff at work or live near a facility that manufactures it, you want to know what it might do. The short answer: Delta-valerolactone sits in a middle ground. Most of the published research doesn’t shout hazard at the top of its lungs, but it asks for respect.
Most chemical safety experts spend long hours poring over toxicology reports and government fact sheets. For delta-valerolactone, the main studies point toward irritation risks. Breathing in the dust or vapor can bug your skin, eyes, and nose. Direct contact sometimes reddens skin. Swallowing a little might make your stomach act up. The U.S. National Library of Medicine matches those takes. Plenty of everyday cleaners can cause the same bunch of problems — so in that sense, it doesn’t rank next to major hazardous substances like concentrated acids or mercury vapor, but safety still deserves attention.
I spent a few college semesters hovering over lab benches and the biggest danger usually hid behind how chemicals looked so plain. Delta-valerolactone is a colorless liquid — it does not look scary at all. That’s how accidents sneak up. A distracted lab partner once mixed it the wrong way and wound up with a nasty rash. The lesson stuck. Just because a chemical looks harmless doesn’t mean it won’t leave a mark.
The big institutions — OSHA, NIOSH, and the European Chemicals Agency — don’t flag delta-valerolactone as a known carcinogen or a reproductive toxin. Safety data sheets (SDS) usually group this compound with chemicals that call for ventilation, gloves, and eye protection. Long-term studies on rats don’t show dramatic toxicity either, but no company should treat it like water. In my own work, that means written procedures, safety goggles, and clear labeling every time.
Among the small crowd dealing with delta-valerolactone, better habits go a long way. Good ventilation is not optional. Neglecting to use gloves is a first step to trouble. Labels should never peel, and training needs to run often. A few bucks spent on personal protective equipment pays off in skin and lung health over time. Sometimes people complain about extra safety steps, yet it usually comes from someone who’s never had a chemical reaction across their skin. I learned to wear long sleeves on every shift after a lesson like that.
Reporting and tracking chemical use matters just as much. Accident logs serve a purpose. People learn best from close calls, as long as someone records what happened. Over the last decade, chemical safety updates helped drive injury rates lower across North America and Europe. There’s still room to grow. People switching jobs or new hires benefit most from seeing exactly what went sideways, not just generic warnings. That’s something every supervisor should remember.
Delta-valerolactone doesn’t make headlines as a silent killer, but even low-profile chemicals need thoughtful handling. I always say: don’t let your guard down around liquids and powders in the lab, keep the gloves nearby, and stay curious about what’s in the bottles. Chemical safety knowledge comes from staying alert — not shrugging off the unknown until trouble comes calling.
Delta-Valerolactone doesn't make front-page headlines, but behind closed lab doors, its hazards loom large. Most people never handle this chemical directly. Researchers and lab techs meet it more often, and careless storage can deliver harsh lessons. A colleague of mine once left a bottle on a sunny bench; the bottle bloated up in a few days, and the air took on a biting, sweet odor. That’s not just a minor inconvenience—a leaky bottle exposes folks to irritation and contamination. Anyone with experience in a chemistry lab will remember at least one incident with a poorly stored volatile like this.
Delta-Valerolactone behaves best in cool, dry places. Warmth invites trouble—heat can build pressure in sealed containers and trigger decompositions. Keeping it below room temperature reduces those risks. The fridge in a well-ventilated chemical storage room, away from food, works for most labs. Preventing direct sunlight and heat sources is more than a guideline; it cuts down on accidental pressure buildup and spoiled stock.
Unopened bottles don’t guarantee safety. This chemical can sneak through weak seals or react with vulnerable plastics. Glass bottles with airtight, chemical-resistant caps give the best protection. Good storage keeps moisture out, stops evaporation, and limits chances of air reacting with the liquid. A cracked cap or cheap plastic can leak fumes, posing risks to workers and compromising experiments. Laboratories and chemical storage guidelines both recommend routine checks—inspecting for signs of swelling, discoloration, or odor keeps everyone safer.
Anyone who’s worked a busy lab knows how easy it is to misplace or confuse bottles. Delta-Valerolactone stores near other organics but shouldn’t cozy up to acids, oxidizers, or strong bases. Those combinations bring fire risks or toxic byproducts. Clear, prominent labeling sidesteps disaster. Warning strips, hazard pictograms, and date-of-opening tags prevent ugly mix-ups, especially during shift changes. Newcomers and seasoned staff both depend on the organization to keep everyone healthy.
Gloves, goggles, and lab coats feel routine until the day someone rushes and skips a step. Cleaning up after a spill isn’t rare. It’s smart to stash some absorbent material and neutralizer nearby, and everyone benefits from regular drills. Fact: the American Chemical Society recommends a detailed chemical hygiene plan including spill management and PPE protocols for all volatile organics. Lax adherence increases both accident rates and insurance headaches.
In my early research years, nobody ever thanked the person who organized the chemical fridge or relabeled aging bottles, but those quiet acts kept the chaos at bay. Proper storage isn’t just about a list of rules. It saves time, protects results, and more crucially, keeps people from getting hurt. Those who remember a bottle bursting, or a persistent chemical odor at the end of the day, learn to take these lessons seriously.
Factories crank out mountains of plastics, fibers, and coatings every year. Delta-valerolactone steps into these assembly lines as a building block. Folks in chemical plants know it as a lactone monomer. They use it to make polyesters and polyurethanes tough enough to handle stress but flexible enough to stretch or bend without cracking. You see these end products in everything from running shoes to phone cases. Delta-valerolactone brings just the right mix of strength and flexibility that designers want for daily-use goods.
Plastic waste keeps piling up, crowding out wildlife and clogging rivers. Many companies now search for better answers. Using delta-valerolactone, polymer chemists shape plastics that break down faster than the old-school kind. The push for compostable food packaging or mulch films owes a lot to this lactone. A big dairy operation might ask for wraps that decompose without polluting soil or harming crops. The shift toward greener plastics owes a debt to the versatility of this molecule.
Drug makers deal with challenges like keeping medicine stable, masked from stomach acids, or floating medicine slowly into a patient’s body. Delta-valerolactone-based polymers help solve these problems. Chemists craft slow-release capsules or hydrogels using this compound. You might visit a clinic and receive a long-acting injectable filled with an active drug bound inside a matrix built from delta-valerolactone chemistry. Doctors appreciate treatments that spread out over time, keeping symptoms in check with fewer trips to the pharmacy.
Ever scraped up against a painted wall and got nothing on your sleeve? Coatings like these owe part of their lasting power to delta-valerolactone. This compound helps link molecules in cross-linked resins, making them more resistant to weather, scratches, and chemicals. On the factory floor, workers might rely on adhesives that keep car parts stuck together, even under vibration and heat. Manufacturers choose delta-valerolactone formulations when they want things to stay strong and hold up under pressure.
Many of the products made today rely on resources pulled from oil. Delta-valerolactone gives researchers hope for using plant-based feedstocks, cutting the tie to fossil fuels. Biotech labs experiment with processes that ferment agricultural waste into this versatile chemical. Lowering costs and scaling up production will take investment, smart people, and plenty of trial and error. Finding clean, affordable ways to make and use delta-valerolactone opens fresh opportunities for innovation, both for growing factories and for anyone who wants cleaner products in everyday life.
People ask a lot from materials: strength, safety, sustainability, and a low price tag. Delta-valerolactone answers some of these demands today, and research teams see even bigger potential down the road. Health, food, and tech companies are already filing patents that bring new twists to how this molecule gets used. Upgrading processes and shifting away from old habits never happens overnight. Progress takes risk, collaboration, and a willingness to rethink what goes into the products that fill up our homes and workplaces. Delta-valerolactone stands out as one of the unsung players guiding chemical innovation in real time.
| Names | |
| Preferred IUPAC name | Oxan-2-one |
| Other names |
Δ-Valerolactone
delta-Valerolactone 5-Valerolactone Oxan-2-one |
| Pronunciation | /ˌdɛl.tə.vəˈroʊ.læk.toʊn/ |
| Identifiers | |
| CAS Number | 542-28-9 |
| Beilstein Reference | 855809 |
| ChEBI | CHEBI:36946 |
| ChEMBL | CHEMBL15811 |
| ChemSpider | 151497 |
| DrugBank | DB02345 |
| ECHA InfoCard | EU: 700-805-6 |
| EC Number | 206-747-7 |
| Gmelin Reference | 7877 |
| KEGG | C04271 |
| MeSH | D006694 |
| PubChem CID | 8810 |
| RTECS number | YD0350000 |
| UNII | 5B5X376F9V |
| UN number | UN2525 |
| Properties | |
| Chemical formula | C5H8O2 |
| Molar mass | 100.115 g/mol |
| Appearance | Colorless liquid |
| Odor | Sweet, coconut-like |
| Density | 1.03 g/mL at 25 °C |
| Solubility in water | Miscible |
| log P | 0.25 |
| Vapor pressure | 0.43 mmHg (25 °C) |
| Acidity (pKa) | 14.7 |
| Basicity (pKb) | Basicity (pKb) : 18.55 |
| Magnetic susceptibility (χ) | -6.08 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.427 |
| Viscosity | 1.94 mPa·s (25 °C) |
| Dipole moment | 4.06 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 276.1 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -589.1 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2511.7 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P210, P261, P280, P305+P351+P338, P304+P340, P312 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | Flash point: 108 °C |
| Autoignition temperature | 215 °C (419 °F; 488 K) |
| Explosive limits | Explosive limits: 1.6–10% |
| Lethal dose or concentration | LD50 oral rat 3200 mg/kg |
| LD50 (median dose) | LD50 (median dose): 800 mg/kg (rat, oral) |
| NIOSH | BO7950000 |
| REL (Recommended) | 25 ppm |
| IDLH (Immediate danger) | No IDLH established. |
| Related compounds | |
| Related compounds |
Gamma-Butyrolactone
Epsilon-Caprolactone Beta-Propiolactone |