
Chemicals like 6-chlorohexanol do not gain widespread attention in large textbooks, but their development marks a clear spot in the growing family of organohalides. Tracing the chemical's roots, chemists in the early 20th century began exploring modifications to simple alcohol structures to test how halogens would alter both reactivity and stability. Synthetic pathways for chloroalkanols started to get refined as researchers saw the chance to introduce versatility into polymers and intermediates. By the 1950s, the need for more precise intermediates in surfactant and pharmaceutical manufacturing gave rise to structured hydrophilic and hydrophobic building blocks. Since then, 6-chlorohexanol became a regular tool for making surfactants, as starting material for biologically active molecules, and as an aid for more complex organic syntheses. The chemical's place today stems from these ongoing efforts to tweak and optimize both industrial scale and laboratory methods.
6-Chlorohexanol belongs to the family of chlorinated primary alcohols. The six-carbon chain gives room for both flexibility and stability, making it more than just a mundane intermediate. Because of the primary alcohol group and the terminal chlorine atom, chemists run into a useful balance between reactivity and manageability. Manufacturers package this compound in metal or HDPE containers, warning about its moisture sensitivity and giving usage suggestions based on storage stability. Compared to its shorter or longer alkyl analogs, it strikes a unique balance in terms of volatility and handling, which keeps it in steady demand.
On the bench, 6-chlorohexanol typically appears colorless to faintly yellow and flows as a slightly viscous liquid at room temperature. Its boiling point hovers near 215-220°C, giving a margin of safety over other chlorinated alcohols that evaporate more quickly. The density clusters around 1.03 g/cm3. Its solubility in water remains moderate for a six-carbon alcohol, but it blends thoroughly in most polar organic solvents. The presence of both an alcohol group and a substituted chlorine means it reacts eagerly with acids, strong bases, and nucleophiles, with correspondingly quick formation of salts, ethers, or even elimination products. The chlorine, attached at the terminal carbon, offers an anchor point for substitution reactions, so modifications become straightforward in skilled hands.
Producers supply 6-chlorohexanol to researchers or industrial customers with technical sheets outlining things like assay (usually ≥98%), refractive index, melting or boiling range, and most common impurities (often minor amounts of diols or chlorohexanone). Labeling includes UN number for safe transport, CAS number (2009-83-8), and safety pictograms following GHS standards. Bulk packaging requires trace impurity analysis by GC or HPLC, and customers check for water content under 0.5% to cut down on hydrolytic degradation. Transport regulations place it in a class where careful handling trumps outright hazard status, but regular inspection against technical sheets remains a standard routine for quality control labs.
Labs and factories often prepare 6-chlorohexanol by controlled chlorination of hexanol or, more commonly, by nucleophilic substitution from 6-chlorohexyl halides and sodium hydroxide in aqueous solution. Another standard synthesis involves treating hex-6-ene with aqueous chlorine, followed by hydrolysis to generate the desired alcohol. Hydrogen chloride and alcohol reaction gives poor yield, so nucleophilic substitution wins most of the time. Not all syntheses match in safety profile: older chlorination routes sometimes release chlorinated byproducts or use solvents that today face stricter regulation. Green chemistry pushes for methods that avoid hazardous byproducts and maximize selectivity, and as the environmental stakes rise, industry adopts multi-step catalysis or phase-transfer catalysts to squeeze out improvements in yield and cleanliness.
Chemists value 6-chlorohexanol for its two reactive handles—the primary alcohol and terminal chloro group—each paving the way for specific chemical modifications. Williamson ether synthesis with alkoxides turns it into ethers. Reaction with sodium cyanide swaps chlorine for nitrile, opening access to amino derivatives through further reduction. Acid treatment can prompt cyclization or elimination under heat, resulting in hexene or substituted epoxides, depending on conditions. The alcohol protects easily via silylation or acetylation, so multi-step syntheses can march forward without unwanted side-reactions at the hydroxyl site. Researchers often cite this adaptability in journals, linking its success with the all-around practical versatility it offers to those pushing toward pharmaceuticals or specialty chemicals.
6-Chlorohexanol wears several labels in global trade: 6-chloro-1-hexanol, 1-Hexanol, 6-chloro-, 6-chlorohexan-1-ol, and sometimes hexanol-6-chloro. Suppliers and database entries (such as ChemSpider or PubChem) use both IUPAC and trivial names interchangeably, which creates some confusion. Those handling purchasing or searching for reference data usually double-check with CAS and structural formula: Cl(CH2)6OH. In documentation, synonyms extend further to reflect salt or ester derivatives, like 6-chlorohexyl acetate or the hydrochloride salt for use in specialized syntheses.
Working with 6-chlorohexanol means wearing gloves, goggles, and often a lab coat as basic protocols. Handling guidelines treat it as an irritant to skin, eyes, and possibly mucous membranes. Spills mean ventilating the workspace and using absorbent pads for cleanup, followed by disposal as chlorinated organic waste. Inhalation guidelines recommend working in a fume hood, especially when heating or distilling. Producers include safety data sheets detailing possible routes of exposure, relevant LD50 values from animal tests, and first aid measures. Real-world safety means up-to-date hazard communication training for staff, routine checks of storage areas for leaks or degradation, and clear SOPs for disposal. Environmental release stays a concern due to persistence of organochlorine compounds, so compliance with local and international regulations frames all phases from shipping to waste management.
More than a simple specialty chemical, 6-chlorohexanol supports production of surfactants, pharmaceuticals, biocides, and polymer additives. Its bifunctional nature grants researchers a high degree of control in building block assembly, particularly for new drug scaffolds or modifying material surfaces for hydrophilicity or reactivity. In textile and plastics, it plays a role as a chain extender or functionalizing agent. Biotechnology teams use it to anchor ligands on surfaces or attach fluorescent groups via easy coupling chemistry. Some pesticide and herbicide syntheses rely on its structure for developing long-chain analogues with targeted properties. Outside core industrial use, academic groups leverage it for small-scale syntheses, probing reaction mechanisms or creating new analytical standards.
R&D teams treat 6-chlorohexanol as a testbed for advancing multi-step synthesis routes. Recent literature explores enzymatic conversions as greener alternatives to traditional halogenation. Efforts go into optimizing yields, boosting selectivity, and minimizing hazardous waste at every stage. Patent filings mention its use for linking hydrophobic alkyl tails to polar heads in new surfactants, and as an intermediate for chain-extended alcohols with pharmaceutical activity. Synthetic chemists publish on how varying reaction partners or conditions can introduce cyclic or branched structures derived from the original molecule. The need for renewable feedstocks for its preparation keeps the spotlight on alternative routes like fermentation or biotransformation, even when scale-up proves tricky.
Studies on 6-chlorohexanol’s toxicity remain a focal point for both regulatory agencies and corporate safety committees. The compound’s acute toxicity hovers around moderate oral and dermal levels, with irritation more common than systemic effects at ambient concentrations. Chronic exposure data remains sparse but animal studies indicate potential central nervous system depression at elevated doses. Metabolism breaks the compound into hexanol and its conjugates, but the chlorine group raises red flags for possible persistent organic pollutants. Ecotoxicity values show moderate concern for aquatic organisms, which pulls regulatory attention to wastewater discharge and accidental release procedures. Long-term epidemiological surveys have not raised widespread alarm, but continued monitoring and animal studies support efforts in risk assessment programs.
Looking ahead, 6-chlorohexanol sits at a crossroads of regulatory, commercial, and scientific interest. Ongoing moves towards sustainability in chemical industry mean future production could favor biobased or less hazardous methods, including enzyme-catalyzed halogenation or electrochemical alternatives. Research pipelines focus on new derivatives for surfactant, pharma, or material science uses, with emphasis on biodegradability and lower toxicity. With advances in analytical methods, tracking environmental persistence and degradation will get easier. As policies tighten, safer practices—like closed-system handling and better waste reclamation—aim to protect workers and local ecosystems while not undermining industrial demand. High-throughput screening and computational modeling may open new uses for the molecule as a lead structure for therapeutic or functional materials discovery, giving 6-chlorohexanol a continued role in both academic and applied research settings.
People rarely hear about 6-chlorohexanol outside of chemistry labs or industrial catalogs, but this modest-sounding compound quietly drives important work in pharmaceuticals and specialty manufacturing. I actually ran into it years ago during a stint at a specialty chemical company, and seeing what it could do opened my eyes to the value of seemingly niche chemicals.
6-Chlorohexanol mainly gets attention from pharmaceutical and fine chemical producers. Laboratories value its six-carbon chain capped with an alcohol group at one end and a chlorine atom at the other. That structure acts almost like a flexible arm in synthetic chemistry, helping scientists assemble molecules that fight infections or treat chronic illnesses. Some newer antiviral agents and drug intermediates use this compound as a starting point.
Access to key intermediates speeds up the research process even for small biotech startups. In pharmaceuticals, the bottleneck rarely lies in discovering a clever idea but in putting that idea together quickly, safely, and in volume. 6-Chlorohexanol steps in right here. Chemists react it with other ingredients to build complicated active pharmaceutical ingredients, sometimes adjusting the chain or swapping the chlorine for different groups depending on the need. Cutting the development timeline by even a few weeks or lowering solvent use translates into cheaper medicines and less strain on manufacturing staff.
Away from pill production, fabric and plastics makers use 6-chlorohexanol to modify polymers. This chemical bonds easily with bigger molecules, letting engineers adjust flexibility or durability in consumer products. For example, 6-chlorohexanol finds a place in certain coatings and performance plastics. You might not spot it by name, but it plays a supporting role in car interiors, wires, or even waterproof items.
Engineers favor it because of its controlled reactivity. They aren’t held back by unpredictable byproducts—something anyone with experience in R&D knows can derail a whole project. Ease of handling makes it practical, offering a balance between efficiency and safety. The need for versatile, reliable intermediates like this has only grown as consumer product regulations demand alternatives to older substances with more health or environmental risks.
Handling 6-chlorohexanol does call for care. In any facility I visited, the right gloves, masks, and room ventilation stood as basic protocols. Safety Data Sheets warn about irritation, and one can't ignore the precaution needed to avoid environmental release. Producers also look for ways to reduce industrial waste during its synthesis, both to comply with tightening laws and to cut costs. Safer supply chains depend on robust transport standards and careful supplier screening.
New trends focus on sustainable chemistry approaches that use renewable sources for base materials, slashing emissions and toxic byproducts. There's plenty of work left here. Universities and startups try biotechnological routes and greener catalysts, hoping to deliver the same pure product with a lighter environmental footprint.
Whether working behind the scenes in life-saving medicine or bringing new features to industrial materials, 6-chlorohexanol has a way of weaving into complex supply chains. My time in the field made it clear: scientific progress pivots as much on these humble chemical tools as it does on big new discoveries. Opening up even a small edge in manufacturing or reliability passes through to patients, workers, and consumers. Every improvement—safety, cost, sustainability—pushes the industry and everyday products a little further ahead.
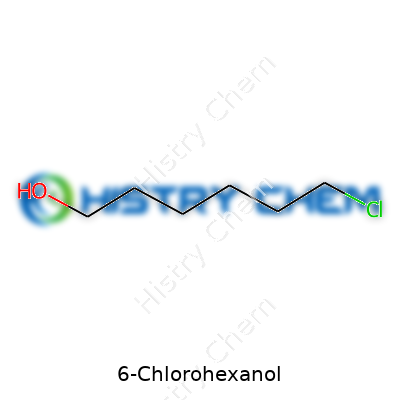
6-Chlorohexanol doesn’t appear on everyone’s radar, but this compound plays a solid role in chemistry labs and industrial synthesis. The chemical formula for 6-Chlorohexanol is C6H13ClO. Knowing this gives a clear starting point for recognizing its structure, properties, and potential uses. If I think back to some long days in college chemistry, untangling these formulas meant breaking the chain—literally. Count out six carbons in a line for the “hex” in hexanol, then tack on an -OH group at the end for the alcohol, and a chlorine atom tagging along at carbon six. That simple label can unlock a deeper understanding of why people care about this molecule.
In research, 6-Chlorohexanol isn’t just a bookshelf curiosity. Its unique structure gives it flexibility in organic synthesis, especially with drug development or crafting specialty surfactants. The chlorine atom gives the molecule extra reactivity. That’s one reason pharmaceutical chemists turn to it when looking for starting materials or intermediates for certain drugs. I remember one project years ago, where a professor had us test different hexanol derivatives in reactions with an eye to see how the placement of a single chlorine would shift the whole game in the final yield and activity. You begin to respect the science behind every label.
Anyone who spends time around chemicals like 6-Chlorohexanol picks up care for detail pretty quickly. The chemical formula guides handling decisions—C6H13ClO tells you a lot. The chlorine atom doesn’t just hang around for show; it can make the compound more reactive and sometimes more hazardous. Long sleeves and gloves aren’t a fashion choice in the lab. I once used a batch of chlorinated alcohols for an undergraduate synthesis—one spill, and that sharp chemical odor taught me fast why ventilation and cleanup matter. Don’t skip material safety data sheets. Always store in well-marked containers and stick to clean handling protocols to keep everyone safe.
Any industry leaning on compounds like 6-Chlorohexanol carries a duty to reduce waste and prevent accidental release. Halogenated compounds often linger in the environment, sometimes tougher to break down, so responsible laboratories invest in good disposal practices. Incineration, not tossing down the drain, keeps population risk far lower. Legislators and regulatory agencies expect strict compliance; the fines for sloppy disposal sting, but the long-term health downsides matter even more. I’ve seen a positive change in recent years—more labs stepping up with cleaner production processes and routine waste audits, driven by both conscience and compliance.
Knowing the structure and formula of compounds like 6-Chlorohexanol shapes real-world progress. Up-and-coming chemists and industrial designers who recognize subtle chemical tweaks—like adding a chlorine here or a methyl group there—keep pushing boundaries in synthesis, medicine, and materials. Building on a strong foundation in basic chemistry opens doors for smarter, safer, and more sustainable technologies. Keeping an eye on both the safety data and the big picture means getting the most benefit with the least harm. Every formula matters because every molecule, sooner or later, winds up touching people’s daily lives.
The chemical world is full of names that slip by most people, and 6-chlorohexanol falls into that group. Its use in research labs and chemical manufacturing may not trigger immediate alarm unless you work with solvents and intermediates. The real question always comes down to safety: can it harm people or the environment?
I remember the first time I worked around halogenated alcohols, chemicals in the same family as 6-chlorohexanol. Our training drilled it in—never touch, never inhale, and always keep gloves on. Products like 6-chlorohexanol often appear in controlled environments for a reason. A single whiff or spill can turn an ordinary day into a scramble for the eyewash station. My nerves never quite calmed until I finished my shift and washed up.
6-chlorohexanol features both a chloro group and an alcohol group on its chain. Chloro-compounds raise eyebrows for toxicologists. Chlorinated organics can damage organs or act as skin irritants. Available safety data indicates that 6-chlorohexanol causes moderate irritation, particularly to the eyes and respiratory system. If you get it on your skin, it can bring redness and discomfort. Swallowing it? That cramping pain in your stomach won't quickly fade. Many chemicals with these features can damage the central nervous system or liver and may have longer-term risks with repeated exposure, though studies specific to this chemical remain limited.
Environmental risk is another part of the story. Chlorinated alcohols tend to persist, especially if they seep into water sources. Substances like these break down slowly and can move up the food chain. Some forms of chlorinated solvents have been linked to harmful effects on aquatic life. Spills that reach drains and make their way to rivers pose a real threat, not just to fish but also to animals and people who rely on those waterways.
Companies and workers rely on up-to-date safety data sheets for every chemical in use. 6-chlorohexanol requires the same attention as other hazardous lab chemicals, even though it might not carry the same high profile. Without personal protective equipment—gloves, safety goggles, proper ventilation—risk of exposure rises quickly. A wipe of a chemical-soaked hand on your face or a splash on your skin can lead to lasting discomfort, and exposure isn’t always limited to the lab. Improper storage or disposal can introduce hazards for janitors, waste handlers, or even families nearby.
Safer practices start with knowledge. Workers who handle 6-chlorohexanol benefit from regular training and clear labeling. Labs and factories must invest in solid ventilation systems. Spills and splashes call for quick cleanup protocols and ready access to neutralizing agents. I always felt safer knowing our team kept spill kits within reach and ran regular drills.
Waste management plays a big part. Never pour leftover chemicals down the drain. Dispose through certified hazardous waste facilities. Laws may vary, but basic care applies everywhere—store tightly closed, away from heat, and far from ignition sources.
As science creates new chemicals and expands their uses, the focus must remain on safety. 6-chlorohexanol, like many chlorinated alcohols, can harm you if mishandled. Up-to-date training and simple caution keep risks low. In my experience, careful planning and respect for chemistry always pay off in the long run. Safety isn’t an extra step; it’s a habit that protects everyone, in and out of the lab.
Working in labs over the years has taught me a lot about respect for chemicals, no matter how basic they seem. 6-Chlorohexanol is no stranger to risk—its structure and reactivity require careful attention. Direct contact can cause irritation, and extended exposure never produces good stories. The substance also tends to give off a sharp scent, signaling a volatility that shouldn't be ignored.
Temperature proves crucial with this chemical. Storing it away from heat sources isn’t just a rule—it’s a must. Heat ramps up the risk for evaporation and reaction. A dedicated chemical refrigerator cuts down on those flashpoints and keeps things stable. It's worth looking out for fluctuations, since unexpected condensation can mess with purity, not to mention safety.
Classrooms and research labs usually rely on glass containers for chloroalkanol chemicals. Glass resists corrosion, and the seal for the lid counts heavily. Leaky lids invite spills, and worse, allow vapors to creep out. Plastic sometimes reacts with certain alcohols, so sticking with borosilicate glass stays safer. Clear labels minimize mix-ups. Two people mistaking flammable solvents have enough stories to fill a book, and none of them end cheaply.
Growing up in a home where paint thinners lived under the sink, I learned the hard way about fumes. 6-Chlorohexanol belongs in a well-ventilated chemical storage area, preferably inside a locked, designated solvent cabinet. These spaces cut down accidental exposure and keep curious hands out. Good air circulation and solid doors mean less risk in the event a bottle cracks or leaks.
One overlooked problem in shared labs is chemical incompatibility. Combining certain storage spaces multiplies hazards—for example, storing strong acids or oxidizers nearby can turn a minor leak into a major reaction. Detailed chemical inventory sheets help keep these hazards apart. Chemists using color-coded shelving or clear separation guidelines rarely face accidents from improper storage.
It’s easy to assume everything’s in order until a sticky patch on the shelf says otherwise. Housekeeping saves hassle. Monthly checks spotting cracked lids, residue, or odd odors let you get out ahead of trouble. Working with cleaning kits and PPE—like gloves and splash goggles—limits fallout if a spill ever happens. Emergency showers and eyewash stations nearby means reacting quickly, not scrambling for help.
Strong safety culture demands training. I’ve watched accidents slow to a crawl when colleagues know the right move for the right chemical. Material Safety Data Sheets belong right alongside these bottles. Employees sign off on reading rules. Brief, direct safety checklists work better than thick binders that collect dust. It's a team effort because mistakes affect everyone in the room.
Investing in modern, fire-resistant storage cabinets pays off, especially as regulations get stricter. Digital logbooks track bottles entering and leaving storage. Quick barcode scans help track shelf life and trigger reminders before products break down or expire.
6-Chlorohexanol, stored right, doesn't need to cause problems. By using knowledge, respect, and vigilance, people build workspaces where harsh chemicals don’t become tomorrow’s emergency.
Handling chemicals like 6-Chlorohexanol isn’t just about ticking off safety rules. Anybody who’s ever carried out practical work in a lab knows accidents rarely occur because people ignore the dangers outright—they often happen when routines get rushed or shortcuts feel tempting. I’ve splashed enough solvents on gloves and stared at suspicious stains on the bench to know that even familiar jobs turn risky if basic habits get sloppy.
6-Chlorohexanol comes with more than a tricky name. It’s an organochlorine, so one has to remember both the alcohol group (which can be a solvent and irritant) and the chloro part (which adds toxicity). Inhaling or getting it on skin can irritate eyes, cause headaches, or bring on nausea. A close friend once forgot to check a glove for even the smallest tear and learned the hard way—no exposure is ever too small to dismiss.
No need to wait for a near miss. Gloves made from nitrile or neoprene handled these types of alcohols and chlorinated compounds better than latex. Safety goggles shield eyes, and lab coats keep chemical splashes off regular clothes. A solid pair of shoes beats open sandals by a mile. I always keep a face shield around for transfers or larger pours. PPE only helps if it actually covers skin and isn’t left unused on the counter.
Any time 6-Chlorohexanol leaves the bottle, it should happen under a fume hood. Even with low volatility chemicals, inhaling vapors—or thinking they’ve all drifted away—is a real risk. I once skipped the hood for a “quick task” and regretted it after my throat started to burn. Stray fumes put everyone nearby at risk, not just the person working with the chemical.
Spills need immediate clean-up with suitable absorbent material—paper towels just spread the liquid around. It pays to know where the nearest spill kit, eyewash station, and emergency shower are. Chemical-safe containers should store both the compound and waste. I had to lead a training in waste disposal once after a colleague tossed contaminated gloves in the wrong bin. All it takes is one overflowing trash can to escalate things fast.
Mistaken identity in the lab—grabbing one bottle instead of another—happens more than folks admit. Everything gets a big, clear label in our workspace. We set out labels showing full names, date received, and hazards. Counting on memory or old notes set someone up for a bad surprise down the road—and chemical burns aren’t forgiving.
Safety meetings and dry runs give newcomers the muscle memory to reach for the right gloves or hit the emergency shower fast. Nobody likes extra meetings, but routine drills mark the difference between minor incidents and long-term health issues.
Basic steps like performing risk assessments before every procedure and inviting another set of eyes to check setups keep the environment safer for everyone. Easy-to-understand safety data sheets should hang on the wall, not sit buried on a hard drive. If something feels off, I speak up; that’s saved me more than once. People make mistakes, but a culture of carefulness corrects them before they get out of hand.
| Names | |
| Preferred IUPAC name | 6-Chlorohexan-1-ol |
| Other names |
6-Chloro-1-hexanol
6-Chlorohexan-1-ol |
| Pronunciation | /ˌsɪksˌklɔːrəʊˈhɛk.sə.nɒl/ |
| Identifiers | |
| CAS Number | [2009-83-8] |
| 3D model (JSmol) | ``` CCCCC(CO)Cl ``` |
| Beilstein Reference | 1207863 |
| ChEBI | CHEBI:85316 |
| ChEMBL | CHEMBL127632 |
| ChemSpider | 10313 |
| DrugBank | DB14005 |
| ECHA InfoCard | 100.031.419 |
| EC Number | 214-253-5 |
| Gmelin Reference | 96923 |
| KEGG | C06509 |
| MeSH | D015544 |
| PubChem CID | 11422 |
| RTECS number | MI8575000 |
| UNII | 97XWD36H13 |
| UN number | UN1993 |
| Properties | |
| Chemical formula | C6H13ClO |
| Molar mass | 150.63 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Odorless |
| Density | 1.05 g/mL at 25 °C (lit.) |
| Solubility in water | miscible |
| log P | 0.88 |
| Vapor pressure | 0.0145 mmHg (25 °C) |
| Acidity (pKa) | 15.5 |
| Basicity (pKb) | 5.48 |
| Magnetic susceptibility (χ) | -8.2 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.454 |
| Viscosity | 2.7 mPa·s (20 °C) |
| Dipole moment | 3.07 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 378.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -234.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3977.7 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | Not assigned |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS06 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P210, P260, P273, P280, P301+P312, P305+P351+P338, P337+P313, P403+P233 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | 104°C |
| Autoignition temperature | 270 °C |
| Explosive limits | Explosive limits: 1.2–7.5% |
| Lethal dose or concentration | LD50 (oral, rat): 1260 mg/kg |
| LD50 (median dose) | LD50 (median dose) for 6-Chlorohexanol: "Rat oral LD50 = 500 mg/kg |
| NIOSH | NA7480000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 6-Chlorohexanol |
| Related compounds | |
| Related compounds |
1-Chloro-6-hexanol
6-Chloro-1-hexanol 6-Bromohexanol 6-Chlorohexanoic acid 6-Chlorohexan-1-ol 6-Fluorohexanol 6-Iodohexanol |