
Walking through the stories of chemical progress, 4’-Hydroxyacetophenone stands as an example of persistence and discovery. Chemists first stumbled upon it in the late 19th century, driven by curiosity around phenolic acetophenones. Early syntheses traced back to efforts in perfumery laboratories, where it became clear that both fragrance and function followed this small molecule. Since then, each decade gave chemists better tools and better understanding, turning crude mixtures into pure crystalline powders. Now, 4’-Hydroxyacetophenone covers both industrial and academic ground, making it a familiar face in chemical stocks across continents.
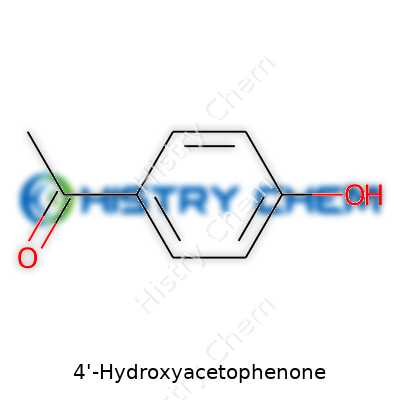
4’-Hydroxyacetophenone presents itself as an off-white, crystalline solid. Its structure, simple yet elegant, shows a benzene ring tagged with both a hydroxy (-OH) and an acetyl group (-COCH3). This set-up gives it access to a wide world of chemical behavior and applications. Suppliers cater to demands from milligram research samples to multi-ton industrial requests. Due to its solid format and moderate volatility, handling remains straightforward for both small and large scale setups.
With a melting point hovering near 109–111°C and low water solubility, 4’-Hydroxyacetophenone likes dry storage and tight bottles. It dissolves much more freely in ethanol, ether, and other organic solvents—making it handy for lab manipulations and blending. The phenolic hydroxyl group plays both donor and participant in hydrogen bonding, which shows up in IR and NMR spectra. Its weakly acidic nature also allows routine deprotonation and alkylation. The crystal structure tends to stack in thin flat plates; this makes weighing and powder handling straightforward, and helps speed up dissolution where needed.
Manufacturers generally ship 4’-Hydroxyacetophenone above 98% purity, with most fine chemical grades pushing past 99%. Typical certificates of analysis include melting point, chromatographic data, and water content by Karl Fischer titration. For regulatory and logistical reasons, labeling practices anchor on chemical formula (C8H8O2), CAS number (99-93-4), and Globally Harmonized System (GHS) pictograms when required. Storage advice stays consistent: cool, dry, and dark containers, well-sealed against moisture and oxidation.
Most industrial methods for making 4’-Hydroxyacetophenone start from phenol or hydroquinone. The Friedel-Crafts acylation route dominates: acetic anhydride or acetyl chloride reacts with phenol, often in the presence of zinc chloride or aluminum chloride as catalysts. The reaction happens in closed vessels, followed by water quenching and distillation. Filtration, washing, and recrystallization fine-tune the product, leaving a solid material free from major contaminants. In research settings, smaller scale syntheses use silica-supported catalysts or green methods with ionic liquids to minimize waste and improve yields.
The two functional groups on 4’-Hydroxyacetophenone give it special leverage in chemical transformations. Chemists enjoy electrophilic aromatic substitution—bromination, nitration, and alkylation come quick, as the hydroxyl directs activity. The acetyl group acts as a handle for reduction or condensation, letting researchers prepare derivatives for new studies. In organic synthesis, the molecule kicks off several condensation reactions, leading to coumarins, chalcones, and related substances. Its hydroxy group undergoes etherification, esterification, and serves as a launching pad for protective group strategies.
4’-Hydroxyacetophenone collects its share of synonyms in catalogues and databases: p-Hydroxyacetophenone, 4-Acetylphenol, and para-Hydroxyacetophenone count as the most popular. Some suppliers add proprietary names or codes, but the backbone always remains the para placement of acetyl and hydroxyl on benzene. During ordering or regulatory reviews, these alternate names draw together, but the chemical identity stands unchanged.
Lab workers appreciate that most handling situations with 4’-Hydroxyacetophenone run without fuss. It falls outside most acute toxicity classifications but deserves routine caution: dust and skin contact can cause irritation. Gloves, splash goggles, and dust masks cover almost all procedures, whether weighing out powder or dissolving in solvent. Large-scale users plan for spills with absorbent material, and disposal routes in compliance with local regulations. Safety Data Sheets include fire-fighting information, since the compound, like many organics, burns as a fine powder in air. Emergency protocols point toward basic first aid—remove contaminated clothing, flush with water, and seek medical help for persistent discomfort.
The reach of 4’-Hydroxyacetophenone spreads far, crossing from pharmaceutical intermediates to cosmetics. In medicinal chemistry, research groups use the compound to build drugs targeting inflammation, infections, or neurological conditions. Aromatic acetophenones hold promise for antimicrobial action, and 4’-Hydroxyacetophenone features often as a scaffold in patent filings. Cosmetic formulations take advantage of its fragrance and mild antioxidant qualities, weaving it into creams and lotions. Material scientists work on new polymers and UV-absorbing compounds, where this molecule takes on both functional and structural roles. Environmental chemists track it as both a breakdown product and a synthetic intermediate, measuring its behavior in soil and water.
In my experience, research around 4’-Hydroxyacetophenone rarely stands still. Each year brings reports of new synthetic methods using greener solvents, recyclable catalysts, and energy-saving conditions. Combinatorial chemists tinker with derivatives, seeking anti-cancer or anti-bacterial activity. Analytical chemists exploit its strong UV absorbance for quality control, and isotope-labeled versions help trace metabolism in living systems. Academics and industry teams partner for high-throughput screens, leveraging the molecule’s reactivity for lead discovery and process optimization. Some projects tackle catalysis or electrochemical sensing, stretching the compound’s footprint into nascent technical fields.
Most published toxicity studies show that 4’-Hydroxyacetophenone does not cause acute harm in lab animals at low doses. Experiments with rodents and cell cultures track both cytotoxicity and long-term effects, looking for signs of DNA damage or mutagenicity. Data from regulatory filings reports no evidence for persistence or significant bioaccumulation, but caution remains for high-concentration use and chronic exposure. Testing in topical products highlights low irritation potential, making it comparatively mild in skin and eye models. Researchers keep watch for any subtle interactions, particularly in the context of complex formulations, food flavorings, or drug precursors.
Looking ahead, 4’-Hydroxyacetophenone continues to attract both established and early-career scientists. Green chemistry advances point toward sustainable production pathways, cutting reliance on harsh reagents and slashing waste streams. Modifications of its core skeleton pave the way for new pharmaceuticals, agrochemicals, and bioactive probes. As disease research tilts toward phenolic and acetophenone motifs, this compound supports both foundational and applied studies. On the industrial side, improved scale-up methods and purification techniques push quality standards higher. Environmental regulators and health agencies will likely revisit exposure standards with new testing data, making regular risk assessment a staple for producers and users alike. The blend of chemical versatility, relative safety, and industrial scalability keep 4’-Hydroxyacetophenone in frequent consideration by chemists and manufacturers seeking practical solutions to real-world needs.
Step into a cosmetics lab and you’ll find scientists reaching for compounds like 4’-Hydroxyacetophenone. It serves as more than just another chemical on the ingredient label. It plays a real role in how products perform and feel on your skin.
Many years spent writing about personal care products taught me one lesson: people want ingredients that are gentle yet effective. 4’-Hydroxyacetophenone works as a skin-conditioning agent and mild antioxidant in a range of skincare items. Unlike some preservatives that cause irritation or dryness, this compound helps stabilize formulas without provoking the same reactions. Anyone who’s dealt with red, blotchy skin after trying new products understands the relief of finding options that don’t trigger flare-ups.
Cosmetic manufacturers rely on steady, stable formulas. Microbial contaminants ruin products before customers get to use them. 4’-Hydroxyacetophenone offers a solution here, bringing both antioxidant and mild preservative effects. It slows down the degradation of creams and lotions by actively neutralizing free radicals. Fewer free radicals mean less oxidation—so the formula feels fresher, and active ingredients stay effective for longer. The safety data for this molecule points towards a low irritation risk, which adds to its appeal.
Beyond the shelves of drugstores and specialty beauty boutiques, 4’-Hydroxyacetophenone surfaces in other sectors. It acts as a building block for pharmaceutical intermediates, which help create medications targeting pain relief, inflammation, and even allergies. Sometimes I wonder how many people, not just doctors and scientists, realize that a compound found in face lotion also helps researchers make new treatments for everyday health concerns.
A fragrance expert once explained how certain molecules form the backbone of scents we recognize. 4’-Hydroxyacetophenone shows up in some flavoring and fragrance blends. Its mild, sweet aroma—slightly reminiscent of almonds—contributes a gentle background note to perfumes and flavored products. It isn’t usually the main character in a scent, but it can anchor and round out complex mixtures. For anyone with a curious nose, tracking the origins of familiar smells often reveals these underappreciated chemical helpers.
Shoppers ask more questions now about where ingredients come from and their safety. Scientists have created greener ways to produce 4’-Hydroxyacetophenone, moving away from harsh reagents and minimizing waste. Transparency matters to those who seek cruelty-free, sustainably produced, and hypoallergenic options. That’s pushed brands to share more about their sourcing and testing methods.
No single ingredient does all the work in skin care or medicine. Still, understanding how something like 4’-Hydroxyacetophenone supports better products helps consumers make smarter choices. Paying attention to the science behind these everyday compounds means fewer surprises for your skin and peace of mind when investing in health and beauty.
4’-Hydroxyacetophenone looks pretty tame if you just read the label. It’s a white, crystalline powder that turns up in labs for all kinds of reasons—flavor agents, pharmaceutical materials, sometimes even cosmetics work. Despite the name not rolling off the tongue, you’d find it all over the industry. Just because it’s common doesn’t mean it should be treated lightly.
I remember the first time I opened a drum of the stuff. Immediately, an instructor stepped in: “Gloves! Goggles!” No room for shortcuts here. Skin can get irritated pretty fast if it gets exposed, and eyes—well, nobody forgets the burn of chemicals in the eye. Nitrile gloves form a better barrier than latex, especially for oily powders like this. Proper safety goggles or face shields make all the difference, and that’s not just the advice of someone who’s seen the Safety Data Sheet. Cutting corners with protective gear leaves you exposed.
If you’re around a lot of powder or you see fine dust kicking up, a particle respirator helps prevent that sharp, chemical scent from becoming a sore throat or worse. Fume hoods make a real difference—breathing in fine organic dust isn’t just unpleasant, it can cause long-term trouble for your lungs. In some research papers, repeated exposure led to respiratory sensitization, which basically means you might end up coughing more than you ever wanted.
Clean work habits keep everyone safe. I learned early to never eat or drink near chemical benches. Chemicals don’t belong anywhere near your lunch, and 4’-Hydroxyacetophenone is no exception. It always lives in a tightly sealed container, stored away from sunlight and away from anything that could set off a reaction. Most lab safety courses push keeping materials off the bench if not in use. Spills happen faster than people expect—cleanup needs to be quick, using proper absorbent materials, followed by a soap-and-water wash for any residue.
Disposing of waste seems tedious until you think about contamination building up. Used gloves, wipes, filter paper—they all go in a marked hazardous waste bin. I saw labs get in trouble for letting this slide: chemical waste mixed with regular trash creates all sorts of headaches for janitorial staff, and leaks can get someone hurt. Waste disposal lines up with local environmental guidelines, which makes sense since the runoff affects everyone. Some companies train their people every six months just to keep these basics fresh.
Standard operating procedures only go so far unless everyone gets good training. In places I’ve worked, the most effective safety came down to simple routines—routine use of gear, good handwashing, smart labeling, and quick reporting if something went off-script. Easy access to eyewash stations and emergency showers adds another layer of peace of mind.
Safety grows stronger when people spot problems and fix them on the spot. If you see powder on the floor, call it out. If a neighbor looks confused by the fume hood settings, offer help. Mistakes show up in places you don’t expect, and it makes a real difference when people look out for each other. One time, a lid came off a bottle halfway through weighing. Instead of freezing up, someone tossed over an extra pair of gloves and we got it cleaned before anyone got hurt. That’s how accidents get stopped: by action, not just by rules on the wall.
In any lab or production setting, the details on chemical quality never deserve to be skimmed over. With 4’-Hydroxyacetophenone, small variances can throw off not only lab results, but also the broader workflow that depends on those results. I’ve watched clients pour time and money into research, only to find inconsistent product quality undo much of their progress. A powder that looks fine on the surface might still harbor hidden contaminants that disrupt a process. This isn’t a risk anyone wants to take—especially when downstream products depend on tight margins for both safety and performance.
Pride in a solid-quality product shows up in purity levels. With 4’-Hydroxyacetophenone, reputable suppliers usually offer powders boasting at least 99% purity, sometimes pushing upwards of 99.5%. These aren’t just numbers for the spec sheet. This sort of clarity means less risk of failures in pharmaceutical development, more reliability in flavor and fragrance creation, and fewer headaches during analytical calibration. Keeping impurities low supports research accuracy and delivers safer outcomes for end users. After one too many delays waiting on a replacement for a subpar batch, I know that batch-to-batch consistency means real peace of mind.
High-quality 4’-Hydroxyacetophenone shows up as a white to off-white crystalline powder with a molecular formula of C8H8O2. From years of handling various organic intermediates, I’ve learned that appearance alone never tells the whole story. Proper melting point (usually 108–111°C) gives another check on identity and purity. For some experienced researchers, an off-spec melting point can signal contamination before any instrument has a say. Moisture content gets measured too, and this figure often lands below 0.5%. Clear, up-to-date Certificates of Analysis (COA)—covering everything from loss on drying to residual solvents—end up being worth their weight in gold in regulated industries.
Purity demands good lab backup. Techniques like HPLC and GC confirm levels of 4’-Hydroxyacetophenone, picking up even tiny impurities. IR and NMR give further confidence. Support data that show no significant levels of heavy metals, halides, or unknown byproducts help companies stick to global standards. After years of field work, I can say that direct access to the latest test reports and genuine batch traceability has saved me from risking project timelines on uncertified chemicals.
Gaps in specification or poor purity hit hard in real-world settings. I’ve seen pharma, cosmetics, and flavor businesses all struggle when their suppliers cut corners. Problems range from batch failures to regulatory violations—issues that go straight to the bottom line and credibility. Regular supplier audits, routine validation of raw material with your own lab, and close reading of the COA each time a shipment lands make a huge difference. Chasing the best price without this level of diligence only leads to setbacks and a higher true cost in the end.
Clear communication with suppliers keeps everyone’s expectations in line—laying out critical purity requirements before placing an order. Certainty comes from seeing specs in writing, down to storage recommendations. Good suppliers support transparency and react fast with documentation. If doubts arise, requesting samples for in-house verification always beats assuming that a certificate tells the full story. Staying proactive means fewer surprises and stronger results, whatever your industry goals.
Not every chemical gets talked about outside the lab, but 4’-Hydroxyacetophenone sits quietly in more places than most folks realize. This compound pops up regularly in organic synthesis, fragrances, and pharmaceutical research. Anyone who’s ever worked with it — or seen a bottle slowly yellow in a back cabinet — knows that storage isn’t just a detail. Safety, purity, and project results all rest on a few smart steps.
Chemicals like to follow their own rules. Sunlight and warmth push 4’-Hydroxyacetophenone toward breakdowns and side reactions. You leave this compound near a window or in a humid corner, and next thing you know, it might start changing color. That’s not just a cosmetic issue. Impurities can sneak into experiments, and lab results go sideways. Storing it away from direct sunlight and keeping it cool isn’t about fussiness. It means you don’t have to second-guess every result, wondering if the reagent is still good.
For anyone who's ever opened a container in a humid room only to spot clumps and discoloration, the lesson sticks: moisture ruins more samples than accidents. 4’-Hydroxyacetophenone absorbs water from the air, so tight seals matter. Personal experience says the extra twist on a lid saves more headaches than it causes, especially in the summer months or in rainy climates. Dry, well-ventilated storage cuts down on that frustration.
Good habits win here. Too many labs feature mystery jars and faded labels. Even the best memory slips, and mistakes don’t stay small for long. Using amber glass bottles instead of clear ones shields 4’-Hydroxyacetophenone from stray UV rays. Plastic sometimes reacts or leaches—glass holds steady. Stick with glass, update the lot number, mark the date the bottle was opened, and your future self will thank you. I’ve seen old containers without labels get tossed out to avoid confusion—money down the drain.
It’s easy to focus on technique and overlook who has access. Chemical misuse makes headlines often for a reason. Lockable storage and tracked access aren’t about inconvenience—they protect people, research, and reputations. In teaching labs or shared spaces, one unlocked cabinet can create a big problem. Even small steps like sign-out sheets or digital logs keep everyone honest and traceable.
Expired or degraded 4’-Hydroxyacetophenone sometimes ends up forgotten. Pushing it to the back of the storage shelf only bumps the risk down the line. Local rules nearly always cover chemical waste for a reason. Let trained disposal teams handle anything past its expiration date or that looks suspicious—no pouring down drains, no trash can shortcuts. Workers, waste handlers, and the environment all stay safer.
Storing 4’-Hydroxyacetophenone doesn’t demand heroics. The best labs run on simple routines: cool, dry shelves out of reach of sunlight; amber glass containers with clear labels and snug lids; secure locations where only approved people get in. These aren’t just checkboxes—they protect hard-won research, keep people healthy, and save on costly replacements. Pay attention up front, and lab work stays a lot less stressful.
People in labs and factories might forget to ask: How long will a container of 4’-Hydroxyacetophenone actually stay useful? Anyone who has handled chemicals knows that shelf life isn’t just a line in a data sheet. It’s the real-world difference between a trouble-free workflow and unexpected setbacks. Picture opening a bottle after a year in storage, only to find a chunky residue and weak performance. That’s more than a minor inconvenience, and it can bleed into lost time and resources.
4’-Hydroxyacetophenone, with its pale crystalline look and its role in flavors, fragrances, and pharmaceutical intermediates, brings stability as an asset for formulators. This compound can last anywhere from two to five years when kept in a sealed container, tucked away from sunlight and moisture. The trick is really in environmental control—room temperature is usually enough, but spikes in humidity fast-track spoilage.
The evidence adds up in chemistry journals and materials safety data sheets—both point to hydrolysis and slow oxidation as the big enemies of quality here. Moisture seeps in, and with time, breaks down the molecule just enough to mess with intended results. Air isn’t innocent either, nudging slow oxidation. Analytical labs catch these down the line through thin-layer chromatography and spectral changes. Clued-in teams set up tracking with date-of-opening labels, a real-world hack that beats wishful thinking.
My years in pharmaceutical labs taught me early: shelf life on paper means less without a real check on the room itself. Old, leaky storage cabinets led to more than one group discovering that their once-bright crystal had turned brown and clumpy after just a year. Handling raw ingredients with gloves, resealing packaging fast, and keeping a logbook for batch tracking transformed our error rate. Organization beat the guesswork. Consistent rotation of stock—using older material first—helped us catch small changes before they created costly surprises.
Problems with lost potency or changed physical appearance rarely begin with the raw chemical itself. Improper containers—thin plastics or damaged glass—invite more humidity and light. Some skip extra desiccant to save pennies, only to face spoiled batches worth much more. Solving these issues comes down to investing in proper storage and training staff to handle chemicals with consistent checks. Of course, manufacturers and vendors should give real retrievable data on shelf life, not shy away from providing batch-specific guarantees.
Users hoping to stretch usefulness without risking product performance need to inspect chemicals before each use. Color, scent, and texture shifts signal that it’s time to retire the batch. Working closely with suppliers, asking about storage recommendations, and sticking to small-batch purchases rather than hoarding big supplies can keep operations smooth. For anyone managing chemicals like 4’-Hydroxyacetophenone, small investments in vigilance pay off, and open communication with suppliers leads to better shelf-life outcomes and safer workspaces.
| Names | |
| Preferred IUPAC name | 1-(4-hydroxyphenyl)ethan-1-one |
| Pronunciation | /ˌfɔːrˌpraɪmˌhaɪ.drɒk.si.əˌsiː.təˈfɛn.oʊn/ |
| Identifiers | |
| CAS Number | 99-93-4 |
| Beilstein Reference | 1908988 |
| ChEBI | CHEBI:78015 |
| ChEMBL | CHEMBL14122 |
| ChemSpider | 6139 |
| DrugBank | DB03848 |
| ECHA InfoCard | ECHA InfoCard: 100.004.018 |
| EC Number | 211-993-6 |
| Gmelin Reference | 82209 |
| KEGG | C01829 |
| MeSH | D000296 |
| PubChem CID | 10755 |
| RTECS number | AM3150000 |
| UNII | LMO8LQ11F2 |
| UN number | UN2811 |
| Properties | |
| Chemical formula | C8H8O2 |
| Molar mass | 136.15 g/mol |
| Appearance | White to off-white crystalline powder |
| Odor | Odorless |
| Density | 1.204 g/cm3 |
| Solubility in water | sparingly soluble |
| log P | 1.26 |
| Vapor pressure | 0.0000527 mmHg at 25°C |
| Acidity (pKa) | 8.05 |
| Basicity (pKb) | 9.38 |
| Magnetic susceptibility (χ) | -50.0 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.546 |
| Viscosity | 1.089 mPa·s (25 °C) |
| Dipole moment | 3.30 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 160.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -322.5 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1963 kJ/mol |
| Pharmacology | |
| ATC code | N02BG09 |
| Hazards | |
| Main hazards | Causes serious eye irritation. |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHSS2=C(C=CC(=C2)O)C |
| Signal word | Warning |
| Hazard statements | H302: Harmful if swallowed. |
| Precautionary statements | Precautionary statements: P262: Do not get in eyes, on skin, or on clothing. P264: Wash hands thoroughly after handling. P280: Wear protective gloves/protective clothing/eye protection/face protection. |
| Flash point | 145°C |
| Autoignition temperature | > 540 °C |
| Lethal dose or concentration | LD50 oral rat 815 mg/kg |
| NIOSH | NA7780000 |
| PEL (Permissible) | No PEL established. |
| REL (Recommended) | 100 mg/mL in DMSO |