
Chemistry has introduced all kinds of compounds that change the way daily life or industry works. 3-Methyl-1,5-pentanediol didn’t get famous overnight. It came out of decades of research into branched diols, back when synthetic intermediates for polymers and resins were still being discovered. Early work focused on uncovering structure-function relationships in glycol molecules, at a time just after World War II when material scientists and organic chemists saw the rising demand for synthetic plastics, resins, and specialty coatings. The compound appeared in patents from research groups hunting for stable, versatile molecules that wouldn’t break down easily at higher temperatures. Back then, a stable diol could mean the difference between a paint lasting years or flaking within months.
3-Methyl-1,5-pentanediol looks like many other diols on paper, but the methyl group in its backbone gives it a unique edge. You’ll find this chemical at the intersection of material science and manufacturing, especially as a building block for special polyesters, plasticizers, and even certain lubricants. As technology moved forward and the demand for more specialized plastics grew, this specific diol became a preferred ingredient partly because it gives finished products a bit more backbone than straight-chain diols. Chemical companies market it in pure, colorless liquid form, shipping it in steel drums or plastic totes, and its applications keep expanding into new areas—from high-performance coatings to plastic modification and even electronics.
With a molecular formula of C6H14O2, 3-Methyl-1,5-pentanediol tips the scales at a molecular weight of about 118.18 g/mol. Its melting point usually sits just below room temperature, and it boils close to 236°C, which means you won’t see it evaporate easily under normal conditions. This diol dissolves pretty well in water, far better than some other branched glycols, and it plays well with many organic solvents. Its flashpoint, coming in above 120°C, tells users right away that storage is less risky compared to lighter, more volatile alcohols. In the lab, its viscosity stands out—it’s thicker than typical glycols, a trait that sometimes determines the end-use application. The molecule’s methyl branch acts like a lock, guarding against rapid oxidation or breakdown, which might otherwise limit its usefulness in harsh chemical settings.
Suppliers deliver 3-Methyl-1,5-pentanediol to customers with color and purity data, often promising over 98% purity, water content well under 0.2%, and minimal traces of color (typically under 10 APHA, which means almost clear). Labels mention not only the product and supplier name, but also batch number, gross and net weight, and clear hazard symbols, because it’s a chemical that still poses health risks when misused. In industrial plants, storage and handling guidelines appear prominently. Documentation will often feature shelf-life info that assures customers of chemical consistency, especially for sensitive applications in medical coatings or electronics. Barcodes help track the drums or bulk containers, making traceability possible from factory gate to end-user site.
Most commercial 3-Methyl-1,5-pentanediol comes from catalytic hydrogenation of the corollary diketone, often produced by aldol condensation of methyl ethyl ketone with formaldehyde. This isn’t a backyard chemistry job; it requires metal catalysts (nickel, copper, or chromium) and closely regulated hydrogen pressure inside fixed-bed reactors. Process engineers watch for temperature control, catalyst poisoning, and product purification—each of which can make or break the batch. Plants recycle and clean up solvent streams to minimize waste. The big advantage of this approach lies in scalability; once a plant dials in these parameters, production can run continuously for days or weeks. Global demand never rivals that of run-of-the-mill glycols, but the process can preserve margins for specialty suppliers.
Chemists appreciate branched diols for their versatility in synthesis. The methyl group at position 3 lets 3-Methyl-1,5-pentanediol take part in reactions that create more robust or specialized molecules. You can etherify, esterify, or even oxidize this molecule into new intermediates—each with its own commercial use. Plastic engineers reach for it when designing polyesters that resist brittleness, and resin manufacturers value its ability to fend off hydrolysis. Certain cross-linking reactions in the lab produce network polymers with higher flexibility, as the methyl branch disrupts crystal packing and lowers melting points. That feature turns out handy in thermoplastic elastomers or hot-melt adhesives. Chemically, the diol participates in all the standard alcohol reactions, forming diesters, carbonates, or even urethanes, depending on which branch of polymer science you’re climbing.
Look through supplier catalogs, and the same compound might pop up as "3-methylpentane-1,5-diol," "3-MPD," or "MPD-3." Some distributors create product labels that refer to it by batch or trademark names, which can confuse anyone who doesn’t double-check the CAS (623-56-3). Trade names sometimes include "Methylpentanediol" or combine the numbers into a brand, though the best practice remains sticking to the IUPAC nomenclature. Research papers may shorthand it as MPD when comparing glycol classes, and companies operating in international markets might assign local names or product numbers for internal tracking.
Labs and factories stress the importance of safety. 3-Methyl-1,5-pentanediol shouldn’t touch bare skin too long, and breathing heavy vapor risks headaches or irritation. Personal experience reminded me how strong an odor some glycols can give off—the smart move is always fresh gloves, splash-proof goggles, and a well-ventilated bench. Safety Data Sheets (SDS) detail the best practices: use chemical-resistant containers, keep away from flames, and store below 30°C. Staff who know their way around chemical handling avoid eating or smoking nearby, and they use spill containment barriers. Disposal calls for incineration or uptake by a licensed chemical recycler. In larger facilities, periodic safety drills ensure everybody knows the evacuation protocol, and all containers should display updated labeling with pictograms and hazard statements in accordance with GHS standards.
Industries welcome 3-Methyl-1,5-pentanediol mostly for its flexibility in manufacturing. Polymer producers value it for creating softer, crack-resistant plastics. Coating developers use it for formulating resins and polyurethane dispersions that need to survive regular abrasion and exposure. Electronics manufacturers pull it into their wiring enamel formulas where insulation flexibility and toughness make the difference between product failure and long-term reliability. As someone who’s worked with specialty adhesive development, the inclusion of this diol can modify performance under stress—a real-world example is heat-activated glue sticks that remain tacky and flexible without charring when the temperature rises. You’ll also find this chemical in lubricants, surfactants, and some solvent blends for high-performance industrial cleaning, thanks to both its solvency and stability.
Academic and corporate research keeps exploring how structural tweaks to glycols influence the next generation of materials. 3-Methyl-1,5-pentanediol gets attention for enabling engineered polyesters that push the limits of impact resistance and weathering. Research groups dig deep into structure-property relationships, testing out novel copolymers or surface coatings with this branched molecule as a key building block. Real-world tests on formulated paints and electronics insulation show how it lengthens product lifespan. Lab teams continue running pilot batches to check for new application areas, while patent literature spins out ideas for greener or lower-energy syntheses. Some groups lean into catalyst optimization, aiming to boost efficiency or reduce the use of expensive metals. My own searches through industry journals consistently throw up new uses tied to its unique backbone, particularly where traditional glycol chemistry can’t quite deliver.
Lab animal testing has given most of the available toxicity profile for 3-Methyl-1,5-pentanediol. Acute exposure rarely causes outright systemic toxicity, though repeated or prolonged contact poses skin and eye irritation in humans, and ingestion in any decent dose leads to gastrointestinal upset. Regulatory reviews show low environmental persistence compared to heavier, halogenated chemicals, which offers some reassurance for responsible users. But chronic exposure data remain thin, so chemical hygiene in manufacturing and R&D stays strict. Toxicologists keep watching for long-term effects, especially with rising production volumes. Environmental labs track breakdown by-products to make sure wastewater doesn’t threaten local waterways, and any new consumer-facing application usually gets a round of extra safety testing.
Material science never stays still, and 3-Methyl-1,5-pentanediol will likely keep finding new applications as industries face stricter safety and sustainability rules. Polymer chemists chase greater performance in everything from flexible electronics to advanced composites, and this compound fits well in the current drive for specialty ingredients that don’t come with harsh environmental baggage. Improvements in green chemistry might soon yield cleaner, lower-carbon production methods. Manufacturing sectors always need molecules with a blend of toughness and processability. As public pressure builds for longer-lasting products and safer, less toxic chemicals, there’s plenty of room for this unsung diol to move out of the shadows and into more mainstream manufacturing, research, or commercial use.
Every time I glance at a product label on a shiny new smartphone or even a water-resistant sneaker, I think about the workhorses keeping those products together and helping them last. 3-Methyl-1,5-Pentanediol isn’t a name most folks know off the top of their head, but it sits behind a wall of innovation in a lot of industries. The main draw? This compound helps make materials sturdier, longer-lasting, and more versatile.
Take paints and coatings, for example. Demand rises for surfaces that don’t easily chip, peel, or lose their shine. 3-Methyl-1,5-Pentanediol is picked for polyester and polyurethane resins, chemicals that make household paints last through messy handprints and rainy days alike. Paint manufacturers appreciate how this diol adds flexibility and helps coatings cling to things like cars, electronics, wood, and even concrete. There’s no magic here—only lots of behind-the-scenes chemistry that ensures the paint you spent three weeks picking out at the hardware store doesn’t start flaking after one season.
Fixing a chair, sealing a bathroom, or holding a big glass panel in place—it’s adhesives all the way down. 3-Methyl-1,5-Pentanediol helps those adhesives stretch and stick. Polyurethane-based glues get a shot of extra durability from this molecule, so they outlast regular all-purpose options. This makes a big difference in places where products deal with rough treatment or temperature swings, such as in construction or car manufacturing.
Think of all the ways plastic sneaks into everyday life. Flooring, insulation, circuit boards, sporting equipment—they all need to hit certain toughness standards. Here, 3-Methyl-1,5-Pentanediol steps in during the making of specialized plastics, giving them flexibility and strength. Tough plastics resist cracks, impacts, and chemical wear, keeping everything from hardhats to children’s toys safe and reliable.
Chemicals like 3-Methyl-1,5-Pentanediol make their way into personal care products too. In lotions, creams, and certain hair products, this ingredient keeps the formula stable and improves texture. It also helps products spread smoothly, which sounds like a minor detail until you try a lotion that clumps or a hair serum that turns sticky overnight. Adding a trusted diol ensures that what comes out of the bottle matches the promises on the label.
These uses matter, but it’s just as important to talk about production and sustainability. Diols usually come from petrochemical sources, and that makes headlines about pollution and resource strain. Chemists and materials scientists are now working on alternatives that start from renewable feedstocks. There’s growing talk of “green” pathways, using biomass or recycled waste, so the footprint shrinks along with each batch produced. Safety remains at the forefront, as industry standards require exhaustive studies about toxicity, environmental impact, and product safety for use in sensitive items like cosmetics.
3-Methyl-1,5-Pentanediol isn’t on most people’s radar, but it has become a linchpin behind paints, coatings, plastics, adhesives, and even a few personal care products. Seeing the full picture brings respect for the chemistry woven into our built world, and underscores how each step toward safer, more sustainable sourcing shapes a smarter, safer future.
There’s always a kind of satisfaction that comes from cracking open the basics of chemistry and finding answers among molecules. For people working with 3-Methyl-1,5-Pentanediol, the chemical formula means more than just letters and numbers on a page—it maps out the backbone of a compound that often winds up in things we use, from coatings to specialty plastics. The formula for this molecule is C6H14O2. That means you’ve got six carbon atoms, fourteen hydrogens, and two oxygens coming together in one chain.
This arrangement isn’t arbitrary. The placement of the methyl group at the third position in the pentanediol chain matters a lot. At the most basic level, chemistry describes a real-world object. I still remember a benchmate in organic chemistry who could rattle off obscure formulas like it was a party trick, but what stuck with me was always the way these small changes in arrangement could completely shift how a molecule acts and feels in an environment. Here, the methyl group not only defines the compound’s name but calls the shots when it comes to physical properties and how it plays with other chemicals.
The molecular weight clocks in at 118.18 g/mol. Some may think this number lives just in the paperwork, but it carries real consequences for how someone uses and transports the material. Every gram counts, especially in manufacturing or in a research setting. I’ve seen how accuracy in weights and measurements stops small mistakes from stacking up into big headaches on the production floor. In pharmaceuticals and fine chemicals, missing the mark on molecular weight affects purity and performance, and costs companies time and money.
Suppliers and end-users keep an eye on the number 118.18 because it underpins everything from dosing and scaling up to preparing safety documentation. During a product specification review with a lab colleague, the difference between theoretical yield and actual yield often came down to tiny, overlooked details like this.
People who work day to day with 3-Methyl-1,5-Pentanediol often interact with the compound in blended formulas or as a key ingredient for polymers and coatings. The molecule offers flexibility for chemists who need a diol with custom reactivity and improved water solubility. Each part of its structure supports those goals. The methyl group brings added hydrophobicity, which finds real-world appeal in areas such as UV-cured resins or even specialty lubricants. As a result, manufacturers can fine-tune end-use performance or address regulatory challenges by using it in small, strategic amounts.
Responsible sourcing and handling grow in importance as global supply chains stretch across borders. Consistent documentation, traceability, and up-to-date certificates of analysis keep everyone from lab techs to environmental health officers on the same page. I’ve watched how companies put their trust in suppliers who show transparency, not just in specifications like molecular weight and formula, but in quality assurance and safety standards. With so many applications on the line, keeping close attention to the details behind C6H14O2 isn’t just academic; it’s about making smart choices in everyday production.
3-Methyl-1,5-pentanediol pops up in chemical catalogs as a specialty alcohol, most often used in coatings, adhesives, plastics, or as an intermediate in synthesis. I’ve come across safety data sheets for a fair share of chemicals in my time. Some give pause, some barely raise an eyebrow. This one lands somewhere in the middle.
Here’s the situation: 3-Methyl-1,5-pentanediol does not leap out as a notorious toxin or an explosion risk. The Global Harmonized System classifies it as a mild irritant. Skin or eye contact brings redness or discomfort in lab studies. Swallowing a small amount by accident does not cause severe poisoning based on animal studies, but it can upset the stomach. Inhaling dust or vapor can make breathing uncomfortable, which is common to most diols. Ignoring the basics — gloves, goggles, lab coat — increases the chance for problems.
OSHA and the EPA do not treat this compound as a particularly tough customer. It is not on the major lists for carcinogenic, mutagenic, or reproductive harm. I checked with several Safety Data Sheets: special permits, air monitoring, or panic-room-style storage aren’t dictated. Still, ignoring simple safety habits would be careless.
Chemists who handle it for synthesis do so in well-ventilated workspaces, most likely under a fume hood. Spills, if they happen, are mopped up with gloves and disposed of in the usual chemical waste stream. Nothing here hints at the dangers tied to things like phosgene or pure alkali metals. That doesn’t give license for slack habits.
Experience shows most accidents come not from notorious chemicals, but from not respecting “routine” ones. This chemical is not lethal, but long exposure, splashes to the eye, or breathing in too much are possible. Someone mixing, bottling, or cleaning labware takes home a warning: don’t mistake “moderate” for “harmless.”
If I had to point to an example, a minor hand rash lasted longer from skipping gloves with a “benign” glycol than anything serious I encountered. Long-term, repeated overexposure for chemicals in its class has caused skin dryness and some allergic reactions, but rarely medical emergencies.
Following best practices always makes sense. That means gloves, basic eye protection, clean workstations, and good ventilation. Equipment for spills and proper labeling also reduces risks. Training helps more than any regulation: people who know what to expect and what to avoid cause fewer accidents. Supervisors who treat every chemical, mild or wicked, with focus set a good precedent.
Government bodies do not slap big warning labels on this diol. Sensible handling brings the greatest safety payoff. No need for high drama or special permits — just smart habits and respect for chemicals. That’s what keeps both workplaces and experimenters safe.
Anyone who has spent time sourcing intermediates or specialty diols for industrial or research work knows there’s a big difference between reading a spec sheet and opening a fresh drum. With 3-Methyl-1,5-pentanediol, what comes out is usually a clear, colorless liquid. Some batches might lean faintly yellow, especially after longer storage, but anything cloudy should raise eyebrows. For most commercial operations, color gives that first clue about quality before numbers come into play.
Commercial samples of this diol usually show up with a stated purity of 98% or higher. Sometimes, suppliers claim ≥99% on their certificates of analysis. Labs spot-check by running gas chromatography, which reveals any hints of related impurities or leftovers from the original synthesis. Purity matters, but so does what’s hiding in that extra percent: residual solvents, water, or even similar diols can mess with epoxies, polyurethanes, or coatings relying on sharp performance.
One reason behind tight specs is how sensitive final products—like specialty plastics—are to impurities here. Even a little bit of extra water can mean foaming, bubbling, or unpredictable curing. That’s why most manufacturers publish water content below 0.1% as a minimum. The substance's boiling point and solubility also demand a high degree of cleanliness, because finishing processes—whether distillation or crystallization—are not forgiving if shortcuts are taken.
In practice, both price and quality swing with demand and where the starting feedstock comes from. Suppliers in Europe and Japan often lead in consistency. Importers from other regions might undercut major brands or offer bulk deals, but experience shows that trace impurities show up more often in these stocks. For a research lab, one off-color or impure drum can cost weeks of troubleshooting. For coatings companies, a bad batch doesn’t just eat time—it can shut down a line.
Bottlers use food-grade or chemical-grade drums and take care to minimize oxygen and water exposure during filling. This helps keep stability high and decomposition low. Packing in nitrogen is common for export shipments to stop color drifting over a few months.
Manufacturers depend on published certificates, but savvy buyers run their own analysis, especially before blending big runs of polyurethanes or polyester-based resins. I’ve seen real-life cases where “acceptable” material had just enough side-products to cause yellowing and slower drying. Chasing down the source required a blend of high-performance liquid chromatography and old-fashioned sniff testing—ethereal, resinous notes crop up in contaminated samples.
Staying on top of quality calls for clear supplier communication and random third-party testing. Real-life relationships matter more than a slick website or a flashy data sheet. Companies that offer technical support, transparency on handling, and strong documentation save partners a world of frustration. Batch retention samples, as simple as they seem, let buyers double-check issues that show up downstream. That level of diligence protects the bottom line and, more importantly, safety and reputation in the long run.
Storing chemicals safely rarely grabs the spotlight, but overlooked details can cause more headaches than most people expect. I’ve worked with warehouses full of industrial chemicals, and experience has shown that just a few degrees or a missed label sometimes spell the difference between a smooth workday and a nightmare of spills or ruined inventory. For 3-Methyl-1,5-Pentanediol, sloppy storage eats away at product quality and could make safe handling trickier down the road.
Direct sunlight, humidity, and open air are not friends with this compound. Always use tightly-sealed containers—HDPE or metal drums with good liners hold up over time. I’ve seen glass get some use, but accidents stack up faster with breakable jars shuffling back and forth on crowded shelves.
Share clear labeling. Durable tags keep confusion and careless mix-ups from creeping in, especially where similar liquids look almost identical. Even during hectic inventory shuffles, sharp labels offer a fast reminder: don’t mix or transfer unless you check twice.
A storeroom set around 20°C keeps most diols in shape. Turn up the heat, and degradation comes on faster—chemists know well what summer heat can do, especially when storage skips climate controls. Humid basements and window-lit rooms train employees in fire drills rather than best storage routines.
I remember one summer we overlooked a spot under a roof vent—condensation after a quick thunderstorm broke down a batch in days. Control humidity as much as possible, using dehumidifiers or proper building insulation. Silica gel packs tossed beside the product help soak up the stray moisture that nearly always sneaks in.
Exposure to air dulls stability. Replace opened containers fast and push out as much air as possible before sealing. Nitrogen blankets often get put to work in large operations, but even for smaller stocks, squeezing out the last bit of air and capping tight cuts down the risk of unwanted changes.
Don’t store 3-Methyl-1,5-Pentanediol near oxidizers, acids, or strong bases. Mixing storage with aggressive chemicals means putting lifespan and safety on the line. One warehouse kept all “glycol-like” substances on a single shelf, but the cleaning crew ended up knocking over incompatible bottles and spent days scrubbing the mess. Stash away from food prep areas and always split storage by hazard class to keep things simple and safe.
Regular inspections spot leaks and swelling containers before they cause real trouble. A log of opening dates and transfer history gives a quick read on which batches should ship out first or need extra attention in quality control.
Safe storage builds more than compliance—it's the backbone of reliable production and a safer workplace for everyone. Time spent creating checklists and keeping the environment steady always pays off in fewer accidents and higher quality output.
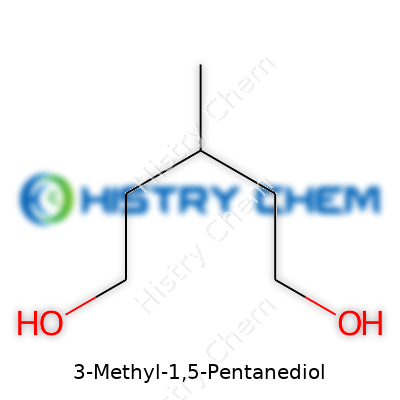
| Names | |
| Preferred IUPAC name | 3-Methylpentane-1,5-diol |
| Other names |
3-Methylpentane-1,5-diol
3-Methyl-1,5-dihydroxypentane 1,5-Pentanediol, 3-methyl- |
| Pronunciation | /ˈθriːˈmɛθɪl waɪn.faɪv pɛnˈteɪn.daɪ.ɒl/ |
| Identifiers | |
| CAS Number | 2568-33-4 |
| 3D model (JSmol) | `JSmol.loadInline("data/mol:CC(C)CCC(O)CO");` |
| Beilstein Reference | 87837 |
| ChEBI | CHEBI:89355 |
| ChEMBL | CHEMBL48979 |
| ChemSpider | 160226 |
| DrugBank | DB08240 |
| ECHA InfoCard | 03e525a0-885f-418c-a585-0e758946f609 |
| EC Number | 214-739-6 |
| Gmelin Reference | 104157 |
| KEGG | C19134 |
| MeSH | D017601 |
| PubChem CID | 11230 |
| RTECS number | SJ5950000 |
| UNII | EC0409P4H0 |
| UN number | Not regulated |
| Properties | |
| Chemical formula | C6H14O2 |
| Molar mass | 120.18 g/mol |
| Appearance | Colorless to light yellow transparent liquid |
| Odor | Mild. |
| Density | 0.937 g/cm3 |
| Solubility in water | Soluble in water |
| log P | 0.5 |
| Vapor pressure | 0.02 mmHg (25°C) |
| Acidity (pKa) | 14.52 |
| Magnetic susceptibility (χ) | -63.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.444 |
| Viscosity | 28.9 cP (25 °C) |
| Dipole moment | 2.56 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 378.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -480.0 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3877.6 kJ/mol |
| Hazards | |
| Main hazards | Causes serious eye irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319 |
| Precautionary statements | P261, P264, P271, P272, P280, P302+P352, P321, P362+P364, P501 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | Flash point: 137°C |
| Autoignition temperature | 280 °C |
| Lethal dose or concentration | LD50 orally in rat: 4600 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 7100 mg/kg |
| NIOSH | SD3700000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 3-Methyl-1,5-Pentanediol is not established |
| REL (Recommended) | 10 mg/m3 |
| IDLH (Immediate danger) | No IDLH established. |
| Related compounds | |
| Related compounds |
1,5-Pentanediol
2-Methyl-1,5-pentanediol 3-Methyl-1,5-hexanediol 2,4-Dimethyl-1,5-pentanediol 1,6-Hexanediol |