
A lot of folks don’t realize how certain niche chemicals thread quietly through the years of organic synthesis. 1,5-Dichloropentane fits that bill. Early literature placed its appearance not long after chloroalkanes captured the attention of early 20th-century researchers. Back then, many of these compounds were simple curiosities in textbooks, but over time, labs around the world started using them as practical intermediates. Versatile bifunctional chlorinated compounds, such as 1,5-dichloropentane, gradually entered organic synthesis textbooks and research papers. Chemists learned to value these molecules for their ability to bridge simple hydrocarbons and highly functionalized products. Chemically, a lot changed with the advances in chlorination technologies; improved safety gear and fume hoods definitely helped people go from minute-scale reactions to work on barrels, so this material became much more accessible for both academic projects and practical manufacturing.
1,5-Dichloropentane doesn’t feature bold colors, but its utility packs a punch in specialized circles. This clear, colorless liquid brings together a modest C5 chain with a chlorine atom on each end. That set-up means both ends of the molecule hold reactive sites suitable for many couplings, substitutions, or even more ambitious ring-closure chemistries. Laboratories and production partners see it as a staple building block, enabling all sorts of product lines — from pharmaceuticals to specialty monomers.
Typical physical specs land somewhere that reminds you this substance means business, not show. 1,5-Dichloropentane boils at around 195°C, with a melting point near −40°C. Its density sits close to 1.07 g/cm³, which means it’s a bit heavier than water but not enough for easy separation. One whiff tells you it carries a sharp, pungent odor — not the kind you forget. Chemically, both terminal chlorines open the door to nucleophilic attack, turning the molecule into a gateway for all kinds of organic transformations, whether you’re after simple ethers or leading down the path to complex ring-closure reactions. Solubility in water stays low, but it dissolves well in many typical organic solvents, which helps during purification and downstream steps.
The drum or bottle a chemist orders arrives with a label that demands respect. Producers specify assay (often 98% or better), water content (typically under 0.5%), and key impurity levels to keep track of side products from chlorination. VOC status and hazardous labeling rarely get overlooked. It’s classified under GHS warnings, with clear pictograms about flammability, corrosivity, and toxicity. Production lots include batch numbers and manufacturing dates, as traceability now falls under strict regulatory eyes. Chemists and techs lean on certificates of analysis to confirm the absence of unnamed contaminants — nobody wants surprises in downstream chemistry.
Synthesis starts with some modest equipment: standard glassware, a reliable source of pentanediol, and anhydrous conditions to control side reactions. Classic methods call for chlorinating 1,5-pentanediol using thionyl chloride, phosphorus pentachloride, or sometimes hydrochloric acid, all under cool, dry atmospheres. Reaction teams run efficient distillation to clean up the product — purity and yield often hinge on the skill of the chemist and how clean the starting diol shows up. Companies with greener ambitions prefer milder chlorinating agents, or continuous processing setups to limit exposure and cut waste, but older literature still favors traditional, direct methods.
This molecule lends itself to all sorts of transformations. Both terminal chlorines stand ready for SN2 displacement, making them prime targets for nucleophiles like amines or alkoxides, letting a chemist produce diamines, diethers, or crown ethers in just a few steps. Cyclization reactions spin these five carbons into rings, useful in pharmaceutical scaffolds. Some polymer chemists use it as a chain extender, slotting its reactive ends into longer, cross-linked networks. If you want to attach a functional group to both sides of a molecule with precision, 1,5-dichloropentane makes the job much more direct. Each step comes with its own quirks; solvents and reaction temperatures matter, and competing eliminations or rearrangements will crop up if you stray from best practices.
Ask a few chemists about this compound and names start flying: 1,5-dichloropentane, pentamethylene dichloride, or even α,ω-dichloropentane. Commercial catalogs sometimes describe it as simply dichloropentane, but this leaves too much room for confusion — after all, four isomers fit that vague title. In academic circles, most stick to the IUPAC name. A quick scan of older safety data sheets might dig up synonyms like pentamethylenedichloride, so careful attention helps avoid botched orders or, worse, mixed-up experiments.
Experience in the lab teaches lessons about safety, and 1,5-dichloropentane comes with its own set of risks. Prolonged skin contact or inhalation leads to irritation, and poorly ventilated spaces can turn noxious fast. Material Safety Data Sheets mandate full gloves, goggles, and well-functioning fume hoods before you pour a drop. Storage in cool, ventilated cabinets keeps vapors under control. Regulations require spill kits, secure disposal methods, and strict inventory controls to prevent environmental release and minimize worker exposure. Facilities handling drums must keep up with local and international shipping codes, given the compound’s flammable and toxic status.
Where chemistry gets creative, 1,5-dichloropentane finds a home. In labs, researchers use the molecule to jump-start syntheses for pharmaceuticals, specialty polymers, and new materials science projects. Its bifunctional design helps bridge carbon chains or ring-close into novel heterocycles. Down the supply chain, production engineers rely on it to manufacture cross-linking agents for resins, active pharmaceutical ingredients, or even select pesticides. Now and then, I hear from colleagues exploring new surfactant constructions or adhesive systems and, sure enough, this compound’s bifunctionality gives them a head start over single-armed building blocks.
Development teams spend plenty of time rethinking the role of 1,5-dichloropentane. Some new patents describe improved catalytic methods for selective substitution, shaving reaction times from days down to minutes. Sustainable chemistry offices ask whether its chlorination route can shift from harsher agents toward milder, less hazardous options, driving down toxic by-products. Analytical groups want tighter specs and real-time QC checks; every improvement in process data, purity, or yield pushes the chemical that much further in high-impact research. Academic groups try out this molecule in synthesis of macrocycles that mimic biological channels or cages for drug encasement. Trials in specialty elastomers and emerging electronics hint at new performance features, like enhanced flexibility or improved chemical resistance, all stemming from its versatile core.
Working with chlorinated solvents and reagents taught generations of chemists a deep respect for their hazards. 1,5-Dichloropentane sits among these, demanding careful handling. Animal studies point toward moderate acute toxicity by ingestion or inhalation, along with high irritation potential for skin, eyes, and mucous membranes. Long-term exposure datasets stay limited, but regulatory authorities urge caution in chronic exposure scenarios. Many schools and research centers restrict its use to closed systems and enforce aggressive PPE standards. Efforts continue to determine biodegradation rates and the potential for persistent residues in soil or water streams. Data gathered feeds into safety training and emergency protocols; nobody in the field wants to relive environmental or health mishaps firsthand.
Looking down the road, the outlook for 1,5-dichloropentane pivots on sustainability, regulation, and ongoing demand for advanced synthesis. Innovation in green chlorination methods promises to lower waste and simplify production, possibly opening the door to broader use in greener manufacturing lines. Polymer scientists keep searching for new ways to harness its structure in high-strength or responsive materials. Regulators in the EU and US review the environmental persistence and expand reporting requirements, so future distribution probably depends on strong product stewardship. That said, for those building new molecules or materials, the lure of reliable terminal chlorines for twin reactions won’t fade soon.
1,5-Dichloropentane comes up in both industrial labs and advanced chemistry classes. It’s a molecule with potential in organic synthesis, often popping up when large companies make specialty materials or pharmaceutical intermediates. So, what’s the formula? 1,5-Dichloropentane’s chemical formula is C5H10Cl2.
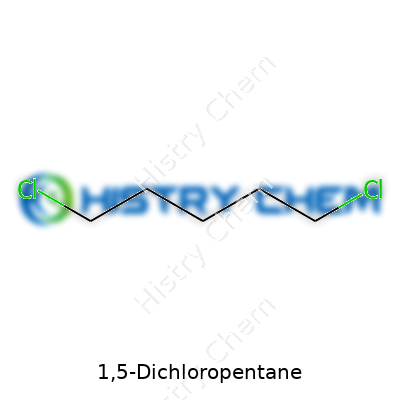
To picture 1,5-Dichloropentane, it helps to imagine a chain of five carbon atoms. Both ends of this chain hold onto a chlorine atom. In simpler terms, there’s a chlorine at position 1 and another at 5, with hydrogens filling up the rest. The chemical backbone looks like this: Cl-CH2-CH2-CH2-CH2-Cl. Pretty straightforward, yet that layout proves useful in many applications.
A lot of people gloss over the structure of organic chemicals, thinking the formula is just theory. In practice, each atom’s spot and the groups they’re attached to steer how the compound reacts. The presence of chlorine atoms on opposite ends of the chain makes this molecule a useful starting point for chemical reactions that need two “handles.” Over the years, I’ve watched labs use compounds like this as bridges between two parts of a new molecule—chemists love a good building block.
If you’ve spent time in research, you see the importance of using substances that match expectations—both for purity and structure. Mixing up 1,5-Dichloropentane with something similar could throw off months of work. Reputable supply catalogs, such as those from Sigma-Aldrich or Merck, list the compound with the C5H10Cl2 formula. NMR and GC/MS analyses back this up, showing clean, repeatable spectra for this structure. People working in chemistry count on consistency, and the experience of generations reinforces the value of sticking to trusted information.
Handling chlorinated hydrocarbons always brings up safety questions. I remember the sharp, almost sweet odor from similar compounds in the lab, an instant reminder to turn on the ventilation. These aren’t chemicals for the casual hobbyist. They’re flammable and can be irritating, and regulations keep a tight eye on their use and disposal. Waste management for dichlorinated compounds means following rules down to the letter, since chlorine atoms can create toxic byproducts if incinerated irresponsibly.
Many places now push for greener synthesis routes, nudging industry and academia to find alternatives with fewer environmental risks. Researchers experiment with milder reagents or swap in less persistent chemicals when possible. This keeps workers safer and the environment cleaner.
Mistakes can happen if care drops, especially in labeling and storage. Double-checking every step in handling and documenting chemicals like 1,5-Dichloropentane can prevent issues. While the structure and formula seem simple enough, there’s a real responsibility in working with chlorinated molecules. Ongoing training, tightening up protocols, and open access to safety data sheets make a difference in day-to-day lab life. It adds up to fewer accidents and more reliable research.
1,5-Dichloropentane’s formula, C5H10Cl2, stands as more than just a set of letters. It represents knowledge, accountability, and a long-running dialogue between chemistry and society.
Anyone who has spent time around chemical labs or within the corridors of a specialty manufacturer knows the value of intermediates like 1,5-dichloropentane. This compound, straightforward as it might appear, often plays a supporting role in many synthesis projects. Years ago, while helping a close friend with his research in organic chemistry, we leaned on this chemical to stitch together more complex molecules. 1,5-Dichloropentane’s real power stems from those two chlorine atoms, spaced just right across the chain, which lets chemists create five-carbon “bridges” in custom molecules.
Drug development leans heavily on this, as chemists searching for new candidates build libraries packed with tailored molecules. A lot of these designs depend on linking chunks together—like combining building blocks for a more advanced toy set. The presence of two reactive ends in 1,5-dichloropentane makes it a favorite in these libraries. Fluorinated drugs, antiviral hopefuls, and even some experimental anti-cancer agents have gone through testing with this molecule somewhere in the recipe.
From my years covering the materials industry, I remember the excitement tied to custom synthetic polymers. The biggest players in automotive plastics and high-durability coatings often source 1,5-dichloropentane for their R&D labs. Those two chlorines can serve as anchor points, letting scientists "stitch" together novel polymers with very specific flex, heat, or resistance properties. This material flexibility supports crucial advances in lighter vehicles, consumer electronics, and specialized medical devices.
Epoxy resins and specialty adhesives count on chemicals like this when tight performance specs must be met. Imagine a manufacturer seeking a clear, heat-resistant glue for advanced electronics—1,5-dichloropentane gives chemists a tool for customizing the "spacer" sections, fine-tuning mechanical and thermal properties much more easily than using the older, one-size-fits-all materials.
Many people overlook the demand for fine chemicals in fields like agriculture and electronics. In crop science, researchers continue to modify pesticide and herbicide molecules to improve effectiveness and reduce environmental impact. Auxiliary reagents such as 1,5-dichloropentane help optimize these molecules by providing “bridges” for desired functional groups. I once interviewed a senior scientist in crop protection who explained how a swap in the backbone, using something just like 1,5-dichloropentane, cut detection time for residues by a third, which matters for food safety and regulatory approval alike.
The electronics sector uses chlorinated intermediates not for the gadgets themselves, but often in cleaners and in certain specialty reagents that process semiconductors. While not as splashy as the big brands, the specialty chemical suppliers behind the scenes keep factory lines moving efficiently, improving electrical component quality by polishing or prepping microchips using such intermediates.
With each benefit, there is always a flip side: safety and environmental issues require continual attention. As a parent, I think about what gets poured down drains or lost in the shuffle at industrial sites. Chlorinated chemicals can persist in soils and water if not contained and treated properly. Companies and labs following Responsible Care protocols invest in better handling, recycling, and less-wasteful processes for 1,5-dichloropentane. Researchers already propose greener synthetic routes, replacing older methods that use excess solvents or create toxic byproducts.
Through smart use, improved training, and a focus on recycling, manufacturers can stretch the value of 1,5-dichloropentane without adding unnecessary risks to people or the planet.
Working with chemicals like 1,5-dichloropentane comes with real responsibilities. This compound brings more than a chemical smell to your workspace—it brings a set of risks you can’t ignore. 1,5-dichloropentane gets used in industrial settings, and even though it isn’t as notorious as some older chlorinated products, it can still threaten your health and the environment if you don’t handle it carefully. I’ve spent years around chemicals, and you start to notice: things only go wrong when someone takes shortcuts. You don’t skip steps when the cost could be your lungs, your skin, or even your job.
Nobody gets points for bravery around toxic substances. I always start my shift by suiting up, and with 1,5-dichloropentane, it means chemical-resistant gloves—nitrile or butyl rubber work best—and safety goggles that seal against the splash. Standard lab coats do a decent job, but a full apron made for chemical work keeps your street clothes—and your skin—safe. Don’t forget about your nose and lungs. If you’re pouring or mixing, a cartridge respirator designed for organic vapors isn’t optional. Just because you can’t smell it doesn’t mean it won’t hurt you.
Concrete floors, big steel drums, and a ventilation fan should be your basic setup. Good airflow makes the difference. I’ve worked in spaces where vapors hang in the air. Open windows won’t cut it—chemical fume hoods or local exhaust systems need to run whenever handling or transferring 1,5-dichloropentane. Don’t compromise on fresh air.
Storage asks for careful thought. I keep the drum tightly sealed, clearly labeled, and away from things like open flames, oxidizers, and anything that could create a spark. Even a small spill can release enough vapor to start a fire or irritate skin and lungs. Chemicals like this don’t store safely alongside acids, strong bases, or anything reactive. A dedicated, well-ventilated chemical store can save a disaster later.
In the lab or the warehouse, hand-to-mouth habits always get people in trouble. Wash up before eating, drinking, or touching your phone. Regular handwashing cuts your risk way down, especially after you finish working with chemicals. Eyewash stations and emergency showers can turn a serious accident into a minor scare, but only if everyone knows where to find them and how to use them. My advice: run a spill or splash drill with your crew, so no one freezes if something hits the fan.
Disposal rules boil down to this: don’t pour anything down the drain or into the trash. Used 1,5-dichloropentane goes into a sealed, labeled hazardous waste container. I talk with the company’s environmental manager or refer to the material safety data sheet for disposal details. Spills stay small if you catch them fast—absorbent pads work in a pinch, but follow up with a full clean and disposal under hazardous waste rules.
No safety policy replaces hands-on training. I’ve seen workers new and old trip up because nobody walked them through proper chemical handling. Refresher training keeps risks top of mind. You want a workplace culture where someone speaks up if something slips—nobody gets left in the dark. Mistakes with chemicals carry a high price, and everybody deserves to go home in the same shape they came in.
Ask someone why boiling points matter, and most folks might think back to high school science. The truth is, boiling points offer more than just trivia—they’re at the heart of working safely and efficiently with chemicals. Take 1,5-Dichloropentane, a clear liquid that’s no stranger to the lab. Its boiling point, which sits around 208°C, isn’t just a number on a label. That figure means something for anyone handling processes from chemical synthesis to waste disposal.
I remember the old lab technician showing me how close observation during distillation could mean the difference between a good yield and an expensive mistake. In the real world, that skill translates into this: look at the boiling point before you turn up the heat. For 1,5-Dichloropentane, that 208°C means you’re dealing with a substance that sticks around as a liquid even as water and plenty of other solvents have long evaporated. This pushes equipment to its limits, especially if your setup isn’t designed for high temperatures.
Anyone working with organochlorine compounds knows these boiling points help predict volatility. A higher boiling point compound like 1,5-Dichloropentane is less likely to vaporize under normal lab conditions compared to lighter solvents. This changes the way you store, measure, and transport it. Safety demands respect for that number. We know inhaling chlorinated vapors isn’t wise, so proper containment and ventilation become essential. There’s nothing theoretical about a chemical’s boiling point once you’ve felt the headache from a poorly ventilated bench. Chlorine’s sharp scent is the warning sign you never want to ignore.
Chlorinated hydrocarbons raise real questions about environmental impact. Boiling points help us predict how quickly something might evaporate or persist in soil and water. With a boiling point over 200°C, 1,5-Dichloropentane tends to stubbornly stick around in the environment unless incinerated. It doesn’t just waft away with a breeze. Regulators look at these numbers closely, setting guidelines for storage to cut down on leaks or improper disposal. Folks in industrial hygiene push for closed systems and tough containers because a spill cleanup isn’t just about mopping up a mess—it’s about protecting people and ecosystems from a chemical that lingers.
Improving chemical safety isn’t rocket science, but it does call for more than just rules. It means checking the data before starting work, knowing at what point a compound shifts from safe to hazardous. In my time, the best teams built their habits around these properties—logging temperatures, reinforcing systems with double-checks, upgrading ventilation when dealing with heavier chlorinated compounds like this one. Training people on what numbers like 208°C mean, not only in theory but in daily practice, goes a long way toward building trust. All the fancy safety measures in the world can’t replace a well-trained set of eyes and hands paying attention to what matters: the numbers that keep us safe and the environment clean.
Ask anyone who’s spent time in a lab or a warehouse, and you’ll get plenty of stories about odd smells, headaches, or worse. Chemicals like 1,5-Dichloropentane make strict storage more than a rule – they make it a matter of real safety. Those who have opened a drum of chlorinated compounds know: improper storage turns small problems big in no time. The chemical doesn’t just ruin shelves; it can slip into your lungs before you know it. That’s not just scary – it’s history repeating itself, since poor chemical storage has caused accidents for decades.
Walking through industrial stores, I’ve spotted drums left by doors or under leaky roofs more often than anyone wants to believe. Storing 1,5-Dichloropentane in a well-ventilated spot, away from anything that can spark, isn’t just common sense – it’s grounded in occupational medicine. Exposure to vapors brings headaches, dizziness, or even bigger health issues down the road. The National Institute for Occupational Safety and Health (NIOSH) recommends limiting exposure based on years of research. Don’t count on sniff tests or open windows to make chemical vapors harmless.
Glass or metal containers with tough, chemical-resistant seals work best. Plastic can turn brittle or leak, especially with chemicals that chew through regular soft seals. My experience tells me that even thick barrels give up eventually if temperature shifts wildly or if the drum sits in direct sunlight. Labeling sounds boring, but it’s burned into my routine after seeing what happens when people mix up chemicals after a shelf cleaning. People work safer, smarter, and more efficiently when every container announces its contents, hazards, and date of first opening.
Nothing builds false confidence faster than a dry, locked storeroom – unless that storeroom heats up in July or freezes in January. Stable temperatures matter. Fluctuations force pressure changes inside containers, sometimes pushing vapors out past a weak seal. I always suggest checking the product’s safety data sheet for specific temperature ranges. Some places install exhaust hoods directly over chemical drums to vent fumes, keeping the air cleaner for everyone.
Static electricity from wool sweaters, sparks from simple light switches, or heat from makeshift coffee pots have all caused fires in chemical storage rooms before. Only use explosion-proof light fixtures and switches in areas where volatile chemicals live. I once watched a coworker reach for a flickering regular lamp, only to have his supervisor jump in and swap it for a certified explosion-safe model. That single move probably saved the business from a broken drum, a fire, or worse.
Even in highly organized facilities, spills sneak in. Spill containment trays aren’t a luxury – they keep drips and leaks from turning into slip-and-fall hazards or environmental headaches. Absorbent pads, neutralizing agents, and a written spill response plan help staff act fast. Watching a trained team address a spill with the right gear beats scrambling after a disaster. Accessible personal protective equipment (PPE) like gloves and goggles doesn’t collect dust in these settings; people reach for them every time.
People who handle and store chemicals need more than printed rules. I’ve seen the difference between a team trained to recognize odors, leaks, or label changes and those left guessing. Real-world stories and hands-on drills push abstract warnings into memory, so mistakes drop off over time. The most experienced worker learns to respect chemicals that “never caused trouble before.”
The Occupational Safety and Health Administration (OSHA) and local environmental agencies review chemical storage policies for good reason – disaster costs more than regular audits or new shelving. The Environmental Protection Agency tracks hazardous spills and waste, and failing to keep their guidelines in mind almost always ends badly. Strong policies that everyone understands save lives, equipment, and the company’s reputation. I’d take regulatory visits and paperwork over health scares and emergency phone calls, any day.
| Names | |
| Preferred IUPAC name | 1,5-dichloropentane |
| Other names |
Pentamethylene dichloride
1,5-Dichloropentamethylene |
| Pronunciation | /ˈwʌn,faɪv daɪˌklɔːrəʊˈpɛnteɪn/ |
| Identifiers | |
| CAS Number | 111-44-4 |
| 3D model (JSmol) | `CCC(Cl)CCCl` |
| Beilstein Reference | 1691340 |
| ChEBI | CHEBI:15705 |
| ChEMBL | CHEMBL155881 |
| ChemSpider | 68208 |
| DrugBank | DB01841 |
| ECHA InfoCard | 01a73208-99a2-4178-bc3a-4d2061677723 |
| EC Number | 203-930-9 |
| Gmelin Reference | 85379 |
| KEGG | C02432 |
| MeSH | D003994 |
| PubChem CID | 12270 |
| RTECS number | SA9100000 |
| UNII | U59N7S011S |
| UN number | UN1993 |
| Properties | |
| Chemical formula | C5H10Cl2 |
| Molar mass | 155.04 g/mol |
| Appearance | Colorless liquid |
| Odor | Sweet odor |
| Density | 1.07 g/mL at 25 °C(lit.) |
| Solubility in water | Insoluble |
| log P | 2.96 |
| Vapor pressure | 0.67 mmHg (25°C) |
| Magnetic susceptibility (χ) | -7.72e-6 cm³/mol |
| Refractive index (nD) | 1.445 |
| Viscosity | 2.807 mPa·s (20 °C) |
| Dipole moment | 2.18 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 309.1 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -147.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3506.8 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02", "GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P210, P261, P280, P301+P312, P304+P340, P305+P351+P338, P312, P337+P313 |
| Flash point | 86 °C (closed cup) |
| Autoignition temperature | 215 °C |
| Explosive limits | Upper: 7.2%, Lower: 1.0% |
| Lethal dose or concentration | LD50 oral rat 2500 mg/kg |
| LD50 (median dose) | LD50 (median dose): 4700 mg/kg (oral, rat) |
| NIOSH | NT8050000 |
| REL (Recommended) | REL (Recommended): 1 ppm (7 mg/m3) |
| IDLH (Immediate danger) | IDLH: 300 ppm |
| Related compounds | |
| Related compounds |
1,3-Dichloropropane
1,4-Dichlorobutane 1,6-Dichlorohexane 1-Chloropentane 2,5-Dichloropentane |