
1,4-Butanediol first turned heads in the chemical industry in the early 20th century, when scientists wanted a flexible compound to bridge gaps in creating polymers and solvents. Built off early research into glycol derivatives, it moved beyond academic curiosity around the 1930s and started gaining traction as companies such as BASF and DuPont looked for workhorse intermediates in the rising plastics sector. Demand grew, not just because of its basic chemical agility, but also because its production relied on relatively accessible feedstocks like acetylene and formaldehyde. Rising innovations in production during the postwar boom tied its story to surging demand for elastic polyurethanes and resilient plastics. The popularity of spandex, flexible electronics, and even certain pharmaceuticals can all tie back to the postwar ramp-up in BDO manufacturing.
Commercial 1,4-butanediol usually flows onto the market as a clear, oily liquid. Its uses range from a major base material for engineering plastics, to thinner applications as a food additive or industrial cleaning solvent. It has a distinctive, somewhat sweet smell. Producers label containers with its CAS number 110-63-4 to avoid confusion, especially since so many derivatives leave the factory floor every day. Private brands sometimes sell it under trade names like Tetramethylene glycol or Butane-1,4-diol. Smart buyers ignore the labels and turn to the paperwork, checking the purity and moisture content, which can shift depending on the manufacturer. Reputable suppliers back up their shipments with technical sheets confirming low levels of impurities, reassurance for downstream users investing thousands of dollars in equipment and labor.
BDO melts just below room temperature, at around 20-21°C. This threshold means it can solidify in cool storage but flows again with a little heat. Boiling starts at around 230°C, placing it far above many common solvents—you won’t see it evaporate off in most real-world environments. BDO’s density sits at about 1.017 g/cm³, and it dissolves with gusto in water, thanks to all those hydroxyl groups. Chemists notice it doesn’t ignite as easily as lighter alcohols, but handle it carefully; it does burn once you reach roughly 200°C, giving off mainly carbon dioxide and water under ideal conditions.
Producers rarely leave room for guesswork. Most BDO shipments reach buyers at over 99.5% purity and often lower than 0.1% water content. Drums and IBCs (Intermediate Bulk Containers) dominate the transport scene. Manufacturers pin explicit hazard labels on each drum, documenting health, fire, and environmental risks. Technical sheets list exact measurements—acidity, residual aldehydes, and trace metals—because each detail influences end-use, particularly in sensitive plastic and pharmaceutical work.
Factories use a couple of key routes for BDO. The classic old-school method starts with acetylene and formaldehyde hitting a copper-bismuth catalyst, pushing through several steps to create the final diol. Industrial players later shifted to butadiene-based approaches, and more recently, direct hydrogenation of maleic anhydride has become popular due to better yields and fewer unwanted byproducts. Each route requires careful control of temperature, pressure, and catalyst composition—a constant balancing act between cost, efficiency, and purity.
Chemists appreciate BDO’s adaptability. Under carefully controlled heat, manufacturers use it to spin up tetrahydrofuran (THF), key for elastic fibers. React it with phosgene, you get polybutylene terephthalate (PBT), a tough engineering plastic. It builds up more complex molecules for pharmaceuticals, textile fibers, and biodegradable plastics. Besides running as a base for polymers, it undergoes selective oxidation, etherification, and other tweaks—some to increase strength or resilience, others to optimize solubility or stability.
Shippers and buyers might call it 1,4-tetramethylene glycol, butane-1,4-diol, or BDO. Europe and Asia sometimes tag containers as 1,4-Dihydroxybutane. Synonyms list keeps growing, but CAS 110-63-4 remains the simplest way to confirm what's getting bought or transported. Some regions still rely on legacy brand names, though standards groups encourage the technical nomenclature for regulatory clarity.
1,4-Butanediol doesn’t belong in just anyone’s hands. Anyone working with it in a plant or lab takes standard precautions: gloves, goggles, masks, and decent ventilation. Neither open flames nor strong oxidizers belong nearby. Plants audit their pipelines for leaks; a single slip-up can send pungent vapors into a workspace. Most countries classify it as hazardous. OSHA insists on training workers in emergency procedures. The chemical’s potential for abuse (as an illicit central nervous system depressant) has sparked tighter controls. Europe and the US, among others, limit its sale to vetted buyers, requiring detailed tracking of movement from plant to end-user.
You’ll find BDO working behind the scenes in dozens of familiar products. Polyurethanes for the auto industry, spandex for clothing, thermoplastic polyesters, solvents for printers and coatings, and even some pharmaceuticals all start their journey here. Without it, you'd see pricier car interiors, less flexible clothing, weaker adhesives, and more fragile electronics. The push for biodegradable plastics leans heavily on BDO derivatives; manufacturers substitute fossil-based diols with partly bio-based versions whenever possible. Electronics makers value its performance as an electrolyte solvent, and medical device makers use it for soft, flexible plastic coatings.
Scientists keep tinkering with both the basic routes and the end-use potential. Academics examine greener synthesis alternatives—think fermentation processes using genetically-tweaked bacteria chomping on sugar—trying to reduce the dependency on oil-based feedstocks. Some labs focus on tweaking catalysts to bump up yield or lower energy costs. Process engineers try to squeeze out trace impurities, aiming for ultrapure BDO for high-end electronics or pharmaceuticals. The research on downstream applications—such as next-generation elastic fibers for sportswear, stronger biodegradable films, or custom pharmaceutical carriers—demonstrates that BDO’s utility keeps expanding.
Workers and regulators know 1,4-butanediol presents risks. Swallowed, it converts in the body to gamma-hydroxybutyric acid (GHB), which depresses the central nervous system—explaining its misuse as a recreational drug. Inhaling or absorbing high concentrations can result in dizziness, vomiting, even unconsciousness. Lab animals exposed to high vapor levels exhibit liver and kidney strain. Precautions should always be at the forefront, especially for people with longer exposure in factories. Animal tests highlight the need for industry monitoring; repeated low-level exposures can build up in fatty tissues, underscoring the value of protective clothing and ventilation systems. Environmental impacts seem moderate, as it biodegrades reasonably well, but accidental spills call for fast clean-up to avoid local waterway damage.
BDO stands on solid ground in manufacturing, but its next chapter will likely favor renewables and stricter oversight. The rising push for plant-based plastics relies on reinventing how BDO gets sourced. More governments tighten controls, hoping to slow down diversion toward illicit drug use, while industry groups champion more automated handling and closed-system transfers to avoid human contact. Expect continuing innovation as companies look for catalysts that cut both energy bills and unwanted byproducts, elbowing fossil fuels out of more product lines. Downstream users lean heavily on emerging research to shrink environmental footprints, all while searching for ways to toughen plastics, stretch fibers, and clean up production streams. For manufacturers and scientists alike, this molecule remains a workhorse—one that draws as much attention for regulation and safety as it does for utility and adaptability.
1,4-Butanediol isn’t a chemical you stumble across in a typical home toolkit, but odds are high that products shaped by it sit close by. Factories tap into the value of 1,4-Butanediol for its strength in making plastics. Think about flexible phone cases, sturdy dashboards in cars, or even athletic gear. Each owes their durability and flexibility to a class of plastics known as polyurethanes and polybutylene terephthalate, which start from this one clear liquid. Demand for these products grows as people expect more convenience and safety from day-to-day items—from water bottles that don’t shatter to lightweight laptops.
Stepping past the factory, 1,4-Butanediol steps quietly into professional workshops that shape spandex and soft fibers. Sportswear stretches, clings, and bounces back thanks to these fibers. Painters know the stuff as a steady base for paints and coatings—making sure colors last through rain and shine. If you checked the label on car cleaning fluids, electronics solvents, or ink used at the local print shop, you’d see the fingerprints of 1,4-Butanediol. It serves almost like a trusted multitool, smoothing out production, making other ingredients mix more easily, and adding a consistent finish.
1,4-Butanediol earned some headlines for all the wrong reasons, too. Used wrongly, this liquid can pose serious health risks. There have been cases where it entered black markets, sold in unregulated products as a so-called recreational compound. Inside the body, enzymes turn it into GHB (gamma-hydroxybutyrate), a powerful depressant. Poisoning, loss of consciousness, and even death show up when people use it far outside industrial walls. The impact stretches past single incidents—hospitals and emergency care centers feel the cost, while law enforcement chases sources that place public health in danger.
The main challenge here isn't just one of chemistry; it comes down to responsible handling. Factories have detailed safety protocols for a reason. Trained workers suit up, and spills get treated seriously. Everyday folks don’t have that gear or know-how. Even a small leak during transport could cause trouble if mishandled. With more people ordering chemicals online, it’s too easy for unsafe products to land in the wrong hands.
A lot rides on stricter oversight. Policymakers and producers both need to keep sharp eyes on how chemicals travel from plants to warehouses to customers. Labels must get clearer, online sales watched more closely, and buyers educated about the real dangers. It comes back to responsible business: train workers, teach customers, and report misuse.
On the bright side, technology stays ahead of trouble more often than not. Digital tracking, improved packaging, and smarter regulations reduce risk without holding back progress. The products shaped by 1,4-Butanediol make lives easier, but keeping everyone safe takes teamwork at each step—from chemical engineers in the lab to people buying end products at the store. Real safety comes from more than rules or warnings; it grows from everyone understanding what’s in each bottle or sheet of plastic, why that matters, and how to handle it right.
Most people never think twice about the clear liquids in industrial drums. 1,4-Butanediol (BDO) is one of those chemicals that seems invisible to the public eye but ends up in so many everyday products. Manufacturers use it to make plastics, elastic fibers, even solvents that sneak into paints and household cleaners. It looks like water and doesn’t carry much of an odor, so you might think it’s harmless. That’s not the truth.
Years in a chemical handling warehouse brought plenty of chances to see BDO being transferred, labeled, and stored. One spill and the room would fill with a faint, sweet scent. Everyone on the floor wore gloves, goggles, even full face shields if a transfer called for it. Any contact with bare skin led to a trip to the safety shower, not just a simple wash at the sink. A drop in the wrong place caused real pain, and left behind a tingling sensation nobody wanted a repeat of.
Medical journals and workplace safety boards agree: BDO isn’t a toy. Once it touches skin, irritation comes next. Inhalation causes dizziness or a scratchy throat; swallowing poses more serious risks, even coma, because the body turns BDO into gamma-hydroxybutyric acid (GHB). GHB is no joke—it’s a controlled substance for a reason. Reports from poison control centers show accidental exposure can lead to hospitalization, and cases involving children and untrained workers have grabbed news headlines.
The National Institute for Occupational Safety and Health puts BDO on lists for substances demanding tight controls. Permanent exposure, even at low doses, can chip away at health. Animal studies hint at liver and kidney stress. No wonder most companies demand workers use chemical-resistant gloves and full coverage clothing.
BDO doesn’t just stop being a problem once it leaves the drum. It dissolves in water, meaning any spill has a path straight into groundwater or local rivers. In a region where one small factory accident years ago left a drinking well unusable, the lesson sticks. Upgrading containment setups to secondary barriers and double-sealed drums wasn’t negotiable anymore. Neighbors paid attention whenever word of a spill hit the local paper.
Straightforward solutions offer the best protection. Real safety doesn’t come just from reading a safety data sheet or watching a training video. Shortcuts invite trouble. Proper ventilation and handling BDO away from food or drinks set the tone. Full-body PPE—thick gloves, goggles, toe-covered shoes—offers a real safeguard, not just a checkmark on a list. Any company that uses BDO in production owes it to workers to supply up-to-date training, not some annual procedural snore session.
Community awareness also plays a part. Strong emergency plans mean everyone knows what to do in case of a spill. Regular drills help spot weaknesses so mistakes get corrected before accidents happen. Local environmental groups can push for tougher spill reporting and support wider monitoring near factories.
BDO brings value in industry, but respect for its power decides safety. Workers, communities, and companies all play a part in reducing risk. That starts with knowledge, sturdy safety habits, and honest conversations about environmental responsibility. Real-world experience with BDO cements one lesson: taking shortcuts with this chemical isn’t worth the risk.
1,4-Butanediol stands out in the world of industrial chemicals. Clear and colorless, this liquid carries a faint, sweet scent. Those who have ever stepped into a petrochemical facility or spent time near a plastics processing line will recognize the subtle but unmistakable whiff in the air. Its physical traits draw attention not just from chemists but from anyone who worries about storage safety, handling, and transport.
1,4-Butanediol melts at 20.1 degrees Celsius. At typical room temperatures, it appears as a thick, syrupy liquid. This low melting point means containers tend to stay liquid under everyday conditions, yet in unheated warehouses in winter, it can thicken significantly or even solidify. Its boiling point hovers around 230 degrees Celsius, which allows for moderate distillation without demanding the heavy engineering required by more volatile substances.
With a specific gravity of about 1.017 at 20 degrees Celsius, its density edges just above that of water. Spills don’t easily float off a loaded warehouse floor; instead, they stubbornly stick, creating more complications during cleanup. Its viscosity feels notably thicker than water—those who pour it during lab work notice the slow, smooth flow. This high viscosity reduces splash hazards a bit, yet extends the time required for tank and pipe evacuation.
What makes 1,4-Butanediol especially interesting is its complete miscibility with water. You can tip any amount into a bucket of water, and the two will blend immediately. This ease of mixing stems from its two alcohol groups. Whether you’re making polyurethane or certain pharmaceuticals, this property speeds up reaction times and simplifies blending procedures. The flip side? Any spill entering drains or surface waters can disperse fast, posing sudden risks to aquatic environments.
Other common solvents easily dissolve this chemical. Ethanol, acetone, and diethyl ether all manage it without issue, making transfer between formulation tanks more straightforward. This high solubility also adds flexibility for cleanups but increases the range of operations where careful chemical management becomes vital.
1,4-Butanediol doesn’t easily ignite. Its flash point sits near 121 degrees Celsius. Compared to more flammable chemicals, the risk of a sudden blaze drops, offering some reassurance for bulk storage and transport. Experienced warehouse technicians notice the lower insurance premiums attached to non-flammable stockpiles. Still, if fires do break out, it may break down to produce strong and hazardous fumes—an important reminder that non-flammable doesn’t mean exempt from danger.
Those working hands-on see the need for insulated or heated storage tanks in colder climates. I remember one particularly cold morning when a pallet of drums became suddenly solid, halting production for hours. Extra equipment for viscosity management always pays off in the long run.
Physical properties matter not just to those engineers building plants or those workers mixing batches, but also to communities sharing space with manufacturing zones. Thinking ahead to water-solubility, spill response, and environmental discharge forms the backbone of any safe plant operation. Better physical understanding has real-world impact—on safety, efficiency, and cost.
1,4-Butanediol shows up in a lot of processes—chemical manufacturing, pharmaceuticals, and plastics all use it. Anyone working with this liquid needs to think about safety before anything else. In my years around chemical shops and factory floors, I’ve seen what happens when somebody skips basic precautions. Spills and fumes put workers at risk and damage equipment, so careful storage isn’t just bureaucracy—it keeps people safe and keeps production running.
This clear, oily liquid isn’t as volatile as some other chemicals, but it reacts if it finds the wrong neighbor or gets too hot. It picks up water from the air and can break down if sunlight or heat gets to it. On top of that, 1,4-butanediol can irritate skin and eyes, and breathing it isn’t good for anyone.
No chemical should be left lying around, but storing 1,4-butanediol takes a few extra steps. Start with the right containers—stainless steel or properly coated drums stop contamination and leaks. Keep them tightly sealed at all times; even a few hours of exposure can spoil a whole batch. Nobody wants to see expensive raw material leak out and gum up a workspace, or worse, end up in a drain.
Temperature control goes a long way. Aim for cool, dry storage away from anything flammable or reactive. I’ve seen one too many storerooms with heat from machinery or sunlight baking the drums—this kind of carelessness shortens shelf life and can get expensive. Use dedicated chemical storage rooms where air circulates but humidity stays low. Don’t stack barrels higher than you can safely reach, and don’t cram incompatible materials nearby—strong acids, bases, and oxidizers belong elsewhere.
According to the National Institutes of Health, 1,4-butanediol may affect the nervous system if fumes build up. OSHA classifies it as having moderate health hazards. I always remind new lab techs that personal protection matters just as much as handling. Goggles, gloves, and splash aprons mean accidents won’t send someone home with a rash or worse.
Routine checks keep surprises away. Look for leaks, drum bulging, or rust. Use proper labeling. Audits keep standards from slipping, especially when staff changes or as supplies move faster than usual. I’ve caught mistakes just from walking past a storage area and noticing pooling liquid under a drum—small catches like that protect everyone.
If a storage area shows its age or if spills are frequent, it pays to upgrade sooner rather than later. Flame-proof cabinets, ventilation systems, and updated spill response kits need investment, but insurance claims and injuries cost a lot more. Training sessions once or twice a year keep safety top-of-mind. Short videos or hands-on demos help lessons stick.
Many companies now use digital inventory tracking to flag old stock or missing drums. Barcode scanning keeps eyes on every batch, so there’s no mystery about what’s on the shelf and no chance of forgotten drums gathering dust in a corner.
Nobody wants to deal with a spill or health scare. Treating storage as a core responsibility makes work safer and keeps production rolling. From my own experience, the companies that get this right avoid breakdowns, keep costs low, and protect their teams. That’s reason enough to get serious about how they store 1,4-butanediol.
1,4-Butanediol pops up in all sorts of industries. Factories churn it out as a solvent, a plastic ingredient, and a rubber hardener. It even finds its way into cleaning products and paints. Despite its usefulness, most people never think about what happens if this chemical ends up in the wrong hands.
Some compounds get a bad reputation not because of what they do on the production line, but because of what people discover they can do outside of it. 1,4-Butanediol is one of them. This chemical breaks down in the body into gamma-hydroxybutyric acid (GHB)—the same GHB that news stories have linked to recreational drug use and abuse. People ingesting or misusing it risk coma, seizures, or even death.
Governments often step in when side effects reach beyond the factory fences. In the United States, 1,4-Butanediol sits in a tricky spot. Federal law does not treat it exactly like GHB, which sits squarely in the list of controlled substances. But as news of overdoses spread, some states decided they could not wait for federal rulemaking. States like California and Georgia decided to regulate or restrict sales and require extra paperwork and controls for people buying large amounts. Other states ask companies to track where and how this chemical moves, to spot anything suspicious long before it becomes a problem in the community.
Europe takes a more unified route. The European Union classifies 1,4-Butanediol as a “drug precursor.” This label triggers extra oversight for companies that make, import, or export this chemical. If you’re a manufacturer there, your paperwork and process get checked by authorities, especially if the shipment heads outside the EU. The United Kingdom, since Brexit, keeps the same standards as the EU—no cutting corners now that borders have more checks.
China’s massive chemical sector deals with 1,4-Butanediol differently. Sales require record-keeping, and the government often keeps an eye on large transactions. Anyone caught diverting this chemical for illicit purposes faces tough penalties.
Rules exist to keep communities safer. A few years ago, headlines about overdoses and party drug use fueled public concern. Politicians and regulators noticed that 1,4-Butanediol could slip through the cracks if laws focused only on GHB. I’ve covered enough local council meetings to know people get nervous once a chemical gets linked to a health crisis. Anyone who has lost someone to overdose gets keenly aware of supply chains and how some products bypass checks.
No law eliminates risk entirely. Smarter regulations can force people to think twice before misusing these chemicals, and push companies to watch their inventory more closely. The balance comes down to not blocking legitimate industry while sealing off avenues for harm. Over the years, companies that handle 1,4-Butanediol have adapted to new rules, installing better tracking and working with officials to keep everything above board.
Policies should evolve with reality. Authorities and retailers need a way to spot patterns—unusual purchasing, missing inventory, or strange requests. I’ve seen businesses invest in digital monitoring tools that flag suspect orders, letting them work with police and turn away bad actors. Training staff helps, too. Newcomers in the chemical business might not spot red flags unless someone teaches them what to look for. Open communication between industry and government makes a difference.
Staying ahead of future risks takes vigilance, honesty, and the willingness to adjust as new problems come up. Communities benefit when both sides—industry and regulators—share responsibility for safety. The spotlight on chemicals like 1,4-Butanediol keeps everyone focused on making choices that protect lives without shutting down industry or innovation.
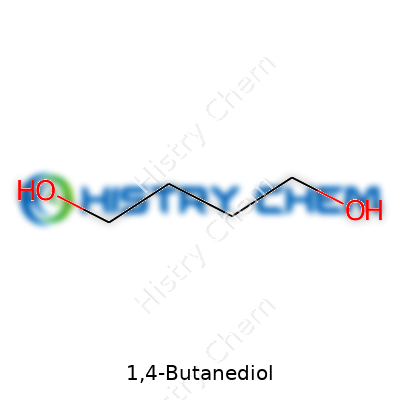
| Names | |
| Preferred IUPAC name | Butane-1,4-diol |
| Other names |
1,4-Dihydroxybutane
Tetramethylene glycol Butane-1,4-diol BDO |
| Pronunciation | /ˌwʌn.fɔːr.bjuːˈteɪn.daɪ.ɒl/ |
| Identifiers | |
| CAS Number | 110-63-4 |
| Beilstein Reference | 1209372 |
| ChEBI | CHEBI:16962 |
| ChEMBL | CHEMBL558 |
| ChemSpider | 8279 |
| DrugBank | DB04453 |
| ECHA InfoCard | 03b6bee7-6803-4866-9cf2-d1be52b51554 |
| EC Number | 203-786-5 |
| Gmelin Reference | 60478 |
| KEGG | C01703 |
| MeSH | D001970 |
| PubChem CID | 8064 |
| RTECS number | UJ9625000 |
| UNII | PCK5D9IV86 |
| UN number | UN 1147 |
| Properties | |
| Chemical formula | C4H10O2 |
| Molar mass | 90.12 g/mol |
| Appearance | Colorless, oily liquid |
| Odor | Odorless |
| Density | 1.017 g/cm3 |
| Solubility in water | miscible |
| log P | -0.96 |
| Vapor pressure | 0.01 mmHg (20°C) |
| Acidity (pKa) | 15.0 (at 25 °C) |
| Basicity (pKb) | pKb: 4.60 |
| Magnetic susceptibility (χ) | -47.2·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.446 |
| Viscosity | 77.3 mPa·s (25 °C) |
| Dipole moment | 2.30 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 104.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -537.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2548.1 kJ/mol |
| Pharmacology | |
| ATC code | V03AB19 |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07,GHS06 |
| Signal word | Danger |
| Hazard statements | H302: Harmful if swallowed. |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P261, P264, P271, P280, P303+P361+P353, P304+P340, P305+P351+P338, P312, P314, P337+P313, P370+P378, P403+P235, P405, P501 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | 135 °C (275 °F; 408 K) |
| Autoignition temperature | 370 °C |
| Explosive limits | 2.0–11.8% |
| Lethal dose or concentration | Lethal dose (LD50, Oral, Rat): 1,525 mg/kg |
| LD50 (median dose) | LD50 (median dose): 1,525 mg/kg (rat, oral) |
| NIOSH | WH7400000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 1,4-Butanediol: Not Established |
| REL (Recommended) | 100 mg |
| IDLH (Immediate danger) | 100 ppm |
| Related compounds | |
| Related compounds |
1,2-Butanediol
2,3-Butanediol gamma-Butyrolactone Tetrahydrofuran Butanediol diglycidyl ether |