
Scientists first paid attention to 1,2-pentanediol as chemical synthesis expanded its reach beyond the academic world and into manufacturing during the late twentieth century. In the years following the Second World War, the chemical industry’s hunger for new compounds drove a wave of research into diols. Around this time, 1,2-pentanediol started drawing interest thanks to its unique structure. Chemists found that its five-carbon backbone and two hydroxyl groups unlocked valuable physical properties, which led to a spread of patents in the late 1950s and into the 1970s. The compound didn’t become a common item on lab benches overnight, but slowly, as new manufacturing methods matured and the price of raw materials dropped, formulations containing this glycol cropped up in cosmetics, plastics, and specialty chemicals.
Producers now offer 1,2-pentanediol as a clear, colorless liquid, which smells faintly sweet. Its straightforward, unbranched carbon chain gives it a sort of simplicity that works well in many recipes. It dissolves easily in water, proving useful for blending with other polar substances. Over the years, chemists have described it as a multifunctional additive, bringing emollient, humectant, and preservative qualities to products. Whether manufacturers needed firmness in cosmetics or a plasticizer in polymer blends, the diol often provided an answer at a reasonable cost.
At room temperature, 1,2-pentanediol remains in liquid form with a boiling point nearing 207°C and a melting point below -35°C. With a density around 0.99 g/cm3 and a molecular weight of 104.15 g/mol, it feels a bit heavier in the hand compared to lighter glycols. The presence of two hydroxyl groups boosts its hydrophilicity and makes it sticky to the touch. The substance resists evaporation and maintains its character over a range of temperatures, soaking up moisture from the air. It blends smoothly with alcohol and water, which helps in many formulations, from lotions to resins.
In regulated markets, standard labels refer to 1,2-pentanediol using several conventions, such as its CAS number 5343-92-0. Packaging usually highlights purity, moisture content, and trace impurity limits. Most chemical suppliers guarantee content above 98%, as manufacturers demand minimal byproducts for sensitive applications. Information sheets list key figures like viscosity, which hovers near 48 centipoise, and refractive index, which sits at about 1.441. As for labeling, regulatory compliance follows standards set by groups like REACH in Europe and the US EPA, since the product enters both industrial and commercial markets.
Today, large-scale producers rely on selective hydrogenation of 2-hydroxypentanal or similar intermediates derived from renewable sources, such as sugar alcohols. Typical processes use metal catalysts—often palladium on carbon—under controlled pressure and temperature. By tuning temperature and hydrogen feed, plants can avoid over-reduction or side reactions, producing batches at more than 90% yield. Labs interested in green chemistry keep searching for ways to use biocatalysts and milder conditions, hoping to scale up environmentally friendly routes in the future.
1,2-pentanediol carries reactive centers at both ends of its molecule—the two hydroxyl groups. This feature encourages chemists to try various modifications. Simple reactions, such as esterification with organic acids, lead to surfactant formation. In the presence of strong acids, it produces cyclic ethers through dehydration. Chemists have also experimented with oxidation and halogenation, hunting for intermediates that could fuel new material designs. Its moderate chain length strikes a balance between volatility and reactivity often required in fine chemical synthesis and advanced polymer science.
The chemical literature lists 1,2-pentanediol under names like pentylene glycol, 1,2-dihydroxypentane, and 1,2-pentylene glycol. Some product catalogs favor the pentylene glycol label, especially in personal care and cosmetics. As a result, label reading becomes essential, since marketing names sometimes hide the IUPAC designation. Suppliers in Asia and Europe sometimes market 1,2-pentanediol for the cosmetics industry under proprietary blend names, though the main ingredient remains consistent worldwide.
Handling 1,2-pentanediol takes less caution than many solvents, but every worker deserves up-to-date information on skin and eye contact. The material’s low volatility reduces the hazard of inhalation, yet usage guidelines still favor gloves and eye protection. Regulatory data from agencies like the European Chemicals Agency highlight a low acute toxicity profile, though extended or repeated exposure brings concerns about irritation. Waste disposal stays straightforward, since the chemical presents no unusual risk to water or land, so long as facilities avoid pouring it down the drain in large quantities. Transport in sealed drums or totes meets UN guidance for non-hazardous liquids.
Beauty brands across the world have adopted 1,2-pentanediol for its moisturizing power and gentle preservative action in skin creams, serums, and cleansers. In these uses, the glycol brings both feel and function, improving product shelf life while softening texture. Polymer manufacturers appreciate its ability to soften resins and manage viscosity in plastics or adhesives. Biomedical researchers experiment with it as a solvent or stabilizing agent in drug delivery systems, tracking how it interacts with both active ingredients and patient tissue. In coatings, 1,2-pentanediol helps balance flexibility with water resistance. Its role expands as researchers push for safer, skin-friendly ingredients that sidestep legacy preservatives with higher allergen risks.
Scientists in both academia and private labs spend considerable energy examining 1,2-pentanediol’s chemistry and testing new synthetic tweaks. Projects seek out environmentally sensitive routes, using enzymes or engineered bacteria to avoid harsh reagents. Research centers carry out detailed analysis of its hydration, mixing, and chemical stress responses. For cosmetics, formulators look for combinations with natural extracts or newer, biodegradable polymers. Fundamental questions revolve around the molecule’s skin penetration, its behavior in water-polymer matrices, and its impact on microbial growth. Industry funding steers research toward solutions that fit the clean beauty movement, as buyers demand traceable ingredients and short, recognizable ingredient lists.
Regulatory dossiers catalog a great deal of toxicological data for 1,2-pentanediol, especially in the last two decades. Oral, dermal, and inhalation studies point to a low toxicity hazard under normal use conditions. Some animal studies found mild irritation at high doses, but overall, the compound clears safety benchmarks set by groups like the FDA and SCCS. Dermatologists still watch for rare cases of sensitivity or contact dermatitis in people with delicate skin, but reports in the open literature stay rare. Regulatory agencies recommend continued monitoring, especially as new uses bring greater contact with consumers, and as stricter safety rules for beauty formulas roll out in Asia and the Americas.
Growth in demand for safer, multifunctional ingredients keeps 1,2-pentanediol on the radar of both researchers and product designers. Chemists chase greener production methods using biomass or waste-derived feedstocks, aiming for a smaller environmental footprint. As more consumers seek out products free from parabens and formaldehyde releasers, this glycol steps up as a preferred alternative, especially for luxury and sensitive skin care. Advances in biotechnology and renewable chemistry could reduce cost and further raise the profile of 1,2-pentanediol as a lower-impact building block. Ongoing studies track its behavior in new delivery systems, especially as interest soars in personalized medicine and high-tech packaging that demands ingredients stable in demanding environments. The need for transparency, reliability, and low hazard profiles places 1,2-pentanediol in a strong position for the coming decade, where both everyday users and technical experts want more from each ingredient inside complex products.
Most people overlook 1,2-pentanediol on product labels. The name sounds like something only a chemist could love, but this liquid finds its way into many items at home and work. If you use skin cream, sunscreen, or even certain types of cleaning sprays, you’ve probably benefitted from this compound. There’s a reason manufacturers continue to rely on it, despite the alphabet soup of ingredients out there promising better results.
One of the big reasons 1,2-pentanediol appears in cosmetics: it attracts moisture. Nobody wants to start the day with a streaky, dry finish after applying lotion. This type of ingredient, known to chemists as a "humectant," helps skin feel smoother for longer. Lower humidity and harsh weather can really do a number on skin, leading to flakiness or tightness. Products with the right kind of moisture boosters cut down on these daily annoyances. I’ve seen this up-close during long, dry winters; products with 1,2-pentanediol left my hands and face comfortable much longer than those with more traditional alcohols or solvents.
Microbes love to find a home in water-based creams and gels, which opens the door to spoilage. Instead of relying on harsh preservatives, companies started looking for ingredients that discourage bacterial and fungal growth without causing irritation. 1,2-pentanediol steps in here. Even at low concentrations, it helps keep formulas fresh, giving users peace of mind the product hasn’t turned into a science experiment. European safety panels have pointed out that it’s gentle for most skin types and doesn’t top the list of common irritants. For people with sensitive skin—myself included—having an ingredient that both hydrates and protects without flaring up a rash makes a real difference.
This compound shows its versatility in other industries, too. Take cleaning products. Some modern disinfectant sprays and wipes use it as a solvent to carry other active agents. Its ability to dissolve a wide range of other ingredients, and its relative safety compared to stronger solvents, led to more adoption in recent years. Paints and coatings sometimes use it for similar reasons. By improving ingredient mixing, manufacturers can cut down on both separation and lumps, creating more reliable products for regular use.
One challenge facing both companies and users involves the ecological footprint of everyday chemicals. Most people don’t realize how some ingredients, even in tiny amounts, affect waterways once washed down the drain. A few environmental studies examined derivatives like 1,2-pentanediol and suggest that it breaks down faster than older, petroleum-based alcohols. It doesn’t linger as long in plants or animals. But that’s not a free pass for unlimited use. Regulations shift as new studies come out, and companies looking for that “green” edge still need to review sourcing and waste.
People want to know more about what they put on their skin and what goes into their environment. I think greater push for transparency, plus stronger oversight from safety panels, will keep ingredients like 1,2-pentanediol front and center. More brands already highlight not just performance but also gentler profiles and lower eco footprints.
As more data rolls in, we’ll all keep weighing convenience, safety, and impact, both for health and the planet. In the meantime, 1,2-pentanediol keeps pulling its weight in lotions, sprays, and way beyond.
1,2-Pentanediol shows up in modern skincare for a reason: it keeps products stable and hydrates the skin. I spot this ingredient on the back of bottles promising soft, clear skin, and for anyone dealing with dryness or sensitive patches, that promise counts. Hydration makes a visible difference, and when the surface feels healthier, confidence tends to follow.
Dermatologists and chemists have checked out 1,2-Pentanediol thoroughly. Studies published in international journals, like the International Journal of Toxicology and reports from the Cosmetic Ingredient Review Expert Panel, found that this chemical causes little to no irritation or allergic reaction in most users. Its gentle profile makes it popular in products meant for kids and adults who struggle with typical irritants.
Clinical testing matters in this context. A large study tested formulations up to 5% concentration and saw almost no adverse effects, even for sensitive skin groups. Yes, some outliers experience redness or itching, but that happens with any new substance. True allergies are very rare. I always tell friends, check ingredients and patch-test, because reactions can never be 100% ruled out with any new product.
Manufacturers like to use 1,2-Pentanediol because it doubles up as a preservative and humectant. It keeps unwanted bacteria away and helps skin hold moisture, a combination that cuts down on extra additives. Parabens earned a bad reputation; as consumers looked for alternatives, this ingredient started to take their place. I prefer minimalist ingredient lists, and 1,2-Pentanediol helps make that possible without risking contamination.
Researchers have also explored its compatibility with other actives. Pairing with vitamin C or niacinamide causes no issues, which widens its appeal across various product types. As someone interested in layering serums and creams, I appreciate ingredients that work well together and don’t cause unpredictable results.
Concerns occasionally crop up around synthetic ingredients in skincare. Some fear build-up or invisible harm over time. Reviews by the European Commission and the US Food and Drug Administration have not flagged any danger at normal concentrations. No scientific evidence supports claims of disruption to hormones or skin’s natural barrier at typical levels.
Anyone worried about synthetic chemicals has alternatives. Products exist that use natural preservatives or focus on oil-based formulas with shorter shelf lives. These tend to feel greasier or spoil faster. I tried several “green” formulations and noticed mold or odd smells before the end of the bottle – so for those not ready to compromise on product stability, 1,2-Pentanediol keeps things safe without harsh parabens or formaldehyde-releasing chemicals.
Safe skincare means understanding both ingredient lists and personal skin history. For most people, 1,2-Pentanediol offers one of the safest bets in moisturizers, cleansers, and sunscreens. The uptime in product safety outweighs the minor risk of irritation for the vast majority.
A patch test makes sense for anyone with an allergy concern. Dermatologists can also help with persistent issues. The search for “clean beauty” continues, but ingredients like 1,2-Pentanediol are helping bridge the gap between performance and peace of mind.
Both 1,2-pentanediol and 1,5-pentanediol pack five carbon atoms and two hydroxyl groups. That sounds similar, but the location of those -OH groups really shapes how each one acts. For 1,2-pentanediol, the hydroxyls settle on the first and second carbon. In 1,5-pentanediol, you find them at the bookends—first and fifth carbon. Looking at their chemical structures, you notice the impact right away.
In the lab, I’ve worked with both. The way each one dissolves and mixes shows you how those positions change the game. 1,2-pentanediol tends to lean toward heavier, slightly oily textures. It’s better at softening plastics, changing viscosity in creams, or acting as a humectant in skin care. 1,5-pentanediol slides more toward water-like feel and fits well in lighter or milder applications, especially in things that need to touch skin often.
A lot of the science turns practical once you look at cosmetics, coatings, or even medicines. 1,2-pentanediol stands out for its antimicrobial punch. It tackles bacteria and fungi, making it valuable as a preservative. That’s why I’ve seen it turn up in fancy moisturizers and wet wipes on store shelves. Small labs and home formulators like it because you don’t have to drown a product in strong-smelling preservatives.
You notice it behaves differently during hot processing or when mixing oil and water. 1,2-pentanediol holds things together a bit more firmly and delivers a thicker, silkier after-feel on the skin. 1,5-pentanediol, on the other hand, takes the lead in baby wipes, eye drops, and solutions needing gentle moisture without stickiness. The placement of its hydroxyls gives it a more neutral scent and lowers irritation risks. I’ve worked on small-batch skin serums where the choice between the two came down to user feedback on texture and comfort.
I always look at safety reports. Both forms are considered safe in low-to-moderate doses. Still, you see some product testing data showing that 1,2-pentanediol, if used at high concentration, may sometimes cause mild skin tingling or redness—nothing alarming for most, but worth noting for folks with sensitive or allergy-prone skin. 1,5-pentanediol tends to avoid trouble with allergies and irritation because of its milder effect on the skin barrier. Data from the Cosmetic Ingredient Review keeps supporting this, which gives smaller and larger manufacturers confidence to switch up ingredients based on the end use.
Some people care about how these chemicals are made. 1,2-pentanediol often comes from more complex synthetic routes, relying on petrochemical inputs or, less commonly, renewable sources. Sourcing and purification drive the price. 1,5-pentanediol, sometimes made by hydrogenating glutaric acid or from biomass, has started to get attention in green chemistry circles. My experience sourcing for eco-friendly labs shows a slight edge for 1,5-pentanediol when buyers push for “greener” branding or certifications.
If you’re trying to pick between the two, look at the balance of safety, feel, function, and supply chain. Both work. Some formulas improve with a swap, while others simply won’t hold together or deliver the right feel unless you make the right choice for that exact use.
1,2-Pentanediol pops up a lot on product labels, especially in skincare and cosmetics. Some people see a long chemical name and feel put off, as if chemicals always mean something artificial or unsafe. The truth, though, is much less alarming. Despite the intimidating name, 1,2-pentanediol is simply a glycol—a type of alcohol with two hydroxyl groups—used for preserving products and helping them stay smooth.
Folks often want to know if something on their skin comes from a plant, an animal, or a lab. That question runs deep in conversations about sustainable sourcing and "clean" beauty. For 1,2-pentanediol, large-scale production relies on synthetic methods. Laboratories usually start with petrochemical feedstocks or work up from more accessible compounds like furfural. A lot of the world’s supply doesn’t come from direct extraction from plants or fermentation, mostly because the chemical isn’t found in abundance in nature.
Plants, fungi, and even some bacteria build a wide range of organic molecules. A few reports suggest 1,2-pentanediol shows up in trace amounts in certain fruits or essential oils, but not in quantities that support mass-market manufacturing. If producers depended completely on natural sources, prices would go up and supply would never keep pace with the booming demand from personal care brands.
I’ve met more and more people who feel uneasy with the idea of synthetic ingredients. Distrust grows out of confusion, and companies often don’t help. Labels throw around words like “natural” or “nature-identical,” but regulations let marketers apply those words to just about anything that started as a plant—even if what’s left at the end of processing barely resembles the original.
Scientific work shows 1,2-pentanediol is safe at concentrations common in cosmetic products. Its main job is to keep bacteria and mold out of your creams and serums. Preservation reduces waste and protects folks from infections. The synthetic version lets this ingredient remain affordable and available, keeping skin-care products on shelves longer without turning them into science experiments in people's bathrooms.
Some chemists see value in developing new fermentation routes, or bio-based syntheses, to make ingredients like 1,2-pentanediol from renewable plant matter. Companies that tap into green chemistry may reach consumers who care about cutting petrochemical use and supporting more transparent supply chains. That shift still needs investment and some tricky problem-solving to match the low cost, purity, and stability of current synthetic routes.
The conversation about natural versus synthetic often boils down to trust and clear information. Ingredients like 1,2-pentanediol don’t change their chemical makeup depending on where they come from. What counts is safe formulation, environmental impact, and honesty about how and why a substance ends up in your daily routine. If companies—and all of us—keep those goals front and center, simple chemistry won’t cause so much controversy.
New ingredients often spark conversations in the world of cosmetics. Buyers scan labels for long, chemical-sounding names and evaluate risks or benefits before letting anything touch their skin. 1,2-Pentanediol, a clear and faintly sweet-smelling substance, keeps surfacing in ingredient lists. Marketers like to call it a “multifunctional” cosmetic additive, but many folks want to know if it effectively protects products from spoilage.
Cosmetic safety draws as much attention as product claims. Preservatives became notorious in consumer debates after concerns about parabens and formaldehyde donors. As brands worked to address public pressure, some ditched traditional chemicals and searched for gentler options that avoid irritation or allergic responses.
Here’s where 1,2-pentanediol gains ground. It offers more than moisturizing perks—it has antimicrobial properties. Reputable studies, including trials published by the Journal of the Society of Cosmetic Chemists, show that at concentrations of around 1–5%, 1,2-pentanediol can slow the growth of bacteria and fungi in formulations like creams and serums. Both small and large skin care labs use it alongside or in place of legacy preservatives.
It’s easy to forget that cosmetics can grow invisible life. Microbes slip in with every scoop or touch, fueled by water and plant extracts inside the jar. Spoiled products not only lose quality—they can trigger breakouts, rashes, or eye infections. Preservatives act as the bouncers at the club, refusing entry to bacteria and mold.
Consumers want safe, effective skin care without irritation. I’ve seen online communities full of folks willing to share photos of bad product reactions. Clean beauty shoppers believe they must trade safety for skin comfort, when truthfully they deserve both. 1,2-Pentanediol gives formulators another tool for broad protection while serving as a humectant to keep the skin barrier healthy.
The science supports careful optimism. Unlike some aggressive preservatives, 1,2-pentanediol breaks down into non-toxic byproducts and ranks low for skin sensitization. Researchers tested its effectiveness in various emulsions, finding better performance when paired with other barrier ingredients. The European Commission’s scientific committee tracks it closely and didn’t flag it for high risk under regulated use levels.
I personally check for well-researched ingredient alternatives if my skin flares up or gets dry. Transparent companies who publish concentration ranges and microtesting data continue to build trust. It’s not about chasing trends, but about making sure safety, performance, and comfort line up. Genuine expertise wins out when labs rely on published research and consumer feedback, rather than greenwashing or vague claims.
Despite clear benefits, using 1,2-pentanediol as a solo preservative isn’t a magic fix. Some stubborn bacteria can still grow in storage, especially in products stored in warm, humid bathrooms. Formula synergy may fall short without the help of additional mild preservatives or chelators. Keeping up with real-world testing beats guessing from lab data alone.
Brands aiming for “minimal” preservative use thrive by letting consumers in on their safety checkpoints. Sharing batch testing, clear expiry dates, and storage tips helps earn consumer loyalty. Ingredient safety hinges on continued monitoring and transparency. Formulators can combine this ingredient with others like ethylhexylglycerin or natural acids, providing double coverage and staying compliant with evolving regulations.
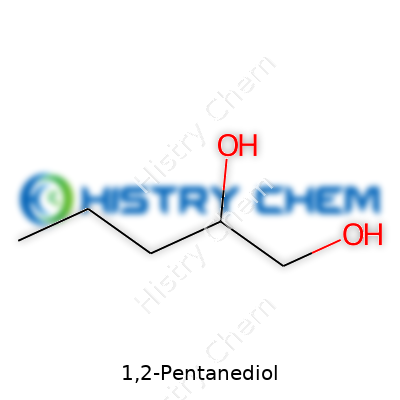
| Names | |
| Preferred IUPAC name | pentane-1,2-diol |
| Other names |
Pentane-1,2-diol
1,2-Dihydroxypentane 1,2-Pentylene glycol Pentylene glycol |
| Pronunciation | /ˌwʌnˌtuːˈpɛn.teɪn.daɪ.ɒl/ |
| Identifiers | |
| CAS Number | 5343-92-0 |
| Beilstein Reference | 1208430 |
| ChEBI | CHEBI:43098 |
| ChEMBL | CHEMBL16248 |
| ChemSpider | 17091 |
| DrugBank | DB16245 |
| ECHA InfoCard | 03b2a8e5-1955-432d-b59d-321d8a3b2f1c |
| EC Number | 01-2119803696-26-XXXX |
| Gmelin Reference | 60462 |
| KEGG | C08297 |
| MeSH | D014867 |
| PubChem CID | 8079 |
| RTECS number | SA8575000 |
| UNII | F44A8PYW7C |
| UN number | UN No. 3082 |
| Properties | |
| Chemical formula | C5H12O2 |
| Molar mass | 104.148 g/mol |
| Appearance | Colorless liquid |
| Odor | Odorless |
| Density | 0.963 g/cm³ |
| Solubility in water | miscible |
| log P | 0.37 |
| Vapor pressure | 0.008 hPa (20 °C) |
| Acidity (pKa) | 14.43 |
| Basicity (pKb) | 15.10 |
| Magnetic susceptibility (χ) | -59.3×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.433 |
| Viscosity | 20.2 mPa·s (25 °C) |
| Dipole moment | 2.67 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 178.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -482.2 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3174.7 kJ/mol |
| Pharmacology | |
| ATC code | D08AX14 |
| Hazards | |
| Main hazards | Causes serious eye irritation. |
| GHS labelling | GHS labelling: "Not a hazardous substance or mixture according to Regulation (EC) No. 1272/2008. |
| Pictograms | GHS06, GHS07 |
| Signal word | Warning |
| Hazard statements | No hazard statements. |
| Precautionary statements | Precautionary statements: P264, P280 |
| NFPA 704 (fire diamond) | 1,1,0 |
| Flash point | 108 °C |
| Autoignition temperature | 210 °C |
| Lethal dose or concentration | LD50 oral rat 4010 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral, rat: 4,500 mg/kg |
| NIOSH | Not Established |
| PEL (Permissible) | No PEL established |
| REL (Recommended) | 1000 ppm |
| Related compounds | |
| Related compounds |
1,3-Pentanediol
1,5-Pentanediol 2,3-Pentanediol 2-Methyl-2,4-pentanediol 2-Pentanol Ethylene glycol Propylene glycol Butanediol |